There are currently 118 total elements (though we may discover more!) Because of this, we use the Periodic Table to sort them all into different rows and columns. Each column, called a “group” contains elements with similar characteristics/properties.
In this article, we will be looking at group 4A and learning all about the different and wonderful elements contained within it!
You are viewing: Which Is The Largest Atom In Group 4a
- This article covers the group 4A elements.
- First, we will learn about the different elements in group 4A and learn where to locate the group on the Periodic Table.
- Next, we will learn about the general trends and properties of the group.
- Then, we will cover the nonmetal of the group.
- Thereafter, we will look at the semimetals/metalloids of the group.
- Lastly, we will cover the group’s metals.
Elements of Group 4A
Let’s begin by looking what what group 4A elements are.
The group 4A elements are the 4th column across when you ignore the transition metals. They are also called the “Carbon family” since carbon is the first of these elements.
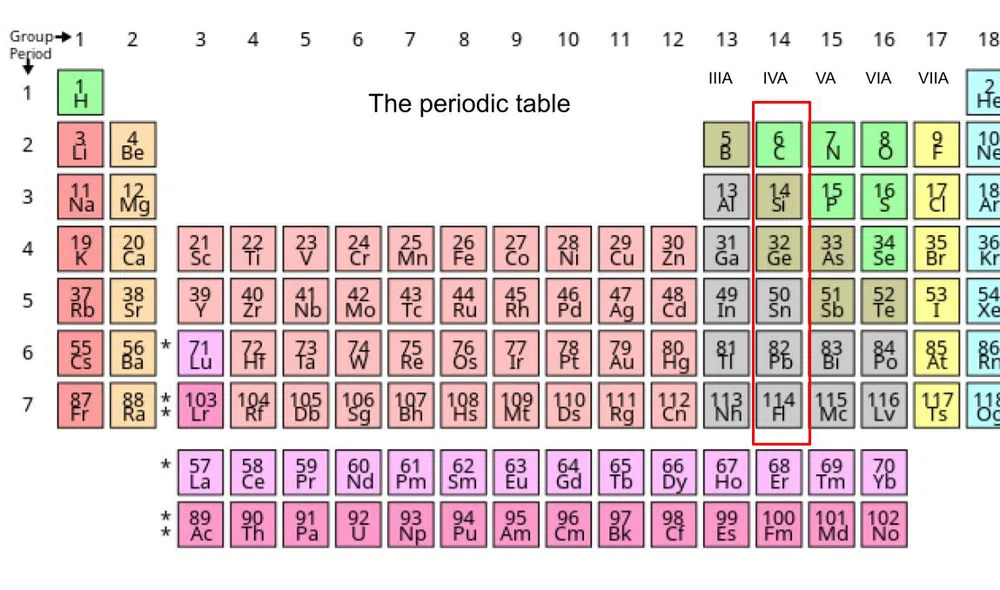
Below is where these elements can be found on the periodic table:
 Fig.1- Periodic table with group 4A marked
Fig.1- Periodic table with group 4A marked
The elements in Group 4A are:
- Carbon (C)-Element 6
- Silicon (Si)-Element 14
- Germanium (Ge)-Element 32
- Tin (Sn)-Element 50
- Lead (Pb)-Element 82
There is another element called flerovium (Fl). It is a man-made element that is highly radioactive. While its properties and reactivity have been studied, the findings are not fully conclusive, which is why we won’t be discussing it much.
Group 4A Properties
Read more : Which Of The Following Would Be A Testable Hypothesis
Group 4A contains elements of all three types: non-metal, metalloid/semimetal, and metal. Because of this, we will be dividing our discussion into sections. However, we are first going to talk about some general properties of group 4A elements.
1. Electron Configuration and oxidation state
-
All the group 4A elements have the same general Electron Configuration for their 4 Valence Electrons: ns2np2.
-
Because of this, they all have the common oxidation state of +4 (lose 4 electrons). However, both tin and lead can have +2 oxidation states, where they lose their p-electrons.
2. Boiling points
-
The boiling points of each element decrease as you down group 4A.
3. Atomic radius
-
The atomic radius is the distance between the center of the nucleus and the outermost electron(s).
-
Read more : Which Best Contrasts Hydroelectric Power And Geothermal Energy
As you move down group 4A, the atomic radius increases.
4. Ionization energy (energy it takes to remove one electron)
-
As you move down group 4A, ionization energy decreases. However, lead’s ionization energy is slightly larger than tin’s
5. Electron affinity
-
Electron affinity decreases as you move down the 4A group.
5. Reactions with hydrogen
-
All group 4A elements react with hydrogen. They generally have the form EH4, where “E” is a group 4A element.
6. Reactions with Oxygen
Source: https://t-tees.com
Category: WHICH