Throughout the rest of this week we will be asking you to evaluate different teaching strategies for helping students get to grips with osmosis, starting with the use of animations in biology, then practical work, and finally modeling. But first, we’ll begin with a quick overview of what osmosis is.
What is Osmosis?
Here’s the definition of osmosis that you will see in most textbooks:
You are viewing: Which Is The Best Definition Of Osmosis
In biology, osmosis is the movement of water molecules from a solution with a high concentration of water molecules to a solution with a lower concentration of water molecules, through a cell’s partially permeable membrane.
A partially permeable membrane (sometimes called a selectively permeable membrane) only allows certain molecules or ions to cross it
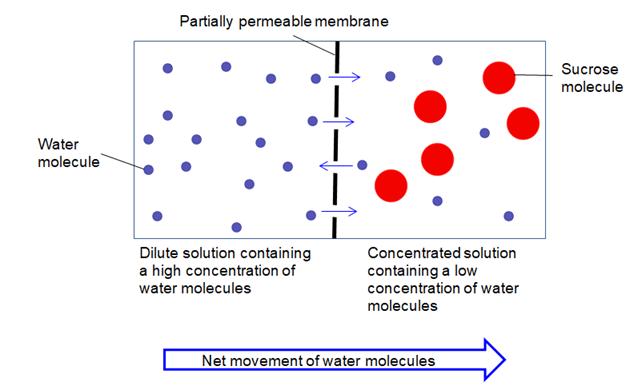
In the diagram above, the higher concentration of water molecules to the left of the partially permeable membrane makes it likely that a large number of water molecules will collide with the membrane and pass through it.
The lower concentration of water molecules on the right-hand side of the partially permeable membrane in the diagram makes it likely that fewer water molecules will collide with the membrane and pass through it.
This means that more water molecules move from left to right on this diagram than move from right to left, and so the overall movement (net movement) is to the right. It is important, though, to stress to students that water molecules are moving in both directions.
Read more : Which Is Not An Application Of Ohm’s Law
You will often see this described as movement ‘down the concentration gradient’, meaning the water is moving from a higher concentration of water (in this case, the dilute sucrose solution), to a lower concentration of water (the concentrated sucrose solution).
If a plant cell is surrounded by a solution that contains a higher concentration of water molecules than the solution inside the cell, water will enter the cell by osmosis and the plant cell will become turgid (firm). The pressure that develops inside a plant cell when it becomes turgid is called turgor pressure. Turgid plant cells help a stem to stay upright.
If a plant cell is surrounded by a solution that contains a lower concentration of water molecules than the solution inside the plant cell, water will leave the cell by osmosis and the plant cell will become flaccid (soft). If the cells in a plant stem become flaccid the turgor pressure inside them will decrease and the stem will wilt.
If a plant cell is surrounded by a solution that contains the same concentration of water molecules as the solution inside the plant cell, there is no overall net flow of water. The movement of water molecules into and out of the cell, through the partially permeable membrane, balances out.
Transpiration Keeps the Water Moving
In plants, water enters the root cells by osmosis and moves into tubes called xylem vessels to be transported to the leaves. Water molecules inside the xylem cells are strongly attracted to each other because of hydrogen bonding (this is called cohesion). When water evaporates from the leaves (through tiny pores called stomata), more water is drawn up from the root xylem cells to replace that which has been lost. A continuous column of water is, therefore, pulled up the stem in the xylem vessels by evaporation from the leaves. This is called transpiration.
Find and share
Source: https://t-tees.com
Category: WHICH
