What is Tollens Test?
Tollens Test is a very useful method to distinguish between aldehydes and ketones. This qualitative lab test is also referred to as the silver mirror test.
Tollens test is generally given by compounds having aldehydic group (aldehydes,alpha-hydroxy ketones and formic acid-its -COOH behaves like a aldehydic group). It gives a white ppt of Silver (where the silver salt is reduced to silver metal and the aldehyde is oxidised to silver salt of carboxylic acid.
You are viewing: Which Of The Following Will Give A Positive Tollens Test
Table of Contents
- Tollens Reagent
- Alpha Hydroxy Ketone Tollens Test
- Tollens Reagent Preparation
- Tollens Test
- Frequently Asked Questions – FAQs
Tollens Reagent
Tollens Reagent refers to the chemical reagent which is used in the detection of an aldehyde functional group, an aromatic aldehyde functional group, or an alpha hydroxy ketone functional group in a given test substance.
The Tollens Reagent is named after Bernhard Tollens, A German chemist who discovered this reagent and its uses. Tollens reagent is a solution of silver nitrate (AgNO3) and Ammonia (NH3).
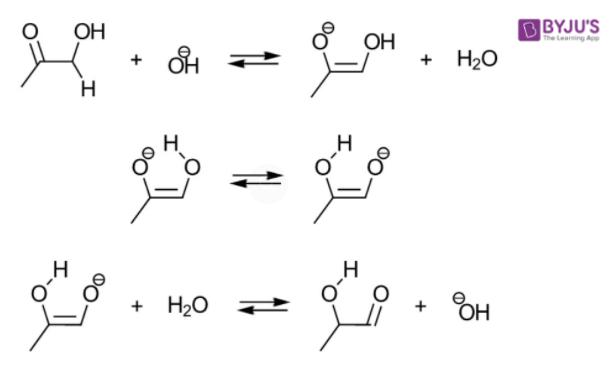
Alpha Hydroxy Ketone Tollens Test
Read more : Which Heathrow Terminal For Virgin Atlantic
Alpha hydroxy ketones are able to give a positive Tollens’ test since α-hydroxy ketones have the ability to tautomerize to aldehydes, and the aldehyde gives the Tollens’ test. An α-hydroxy ketone that cannot tautomerize to a aldehyde won’t give a positive Tollens’ test, like benzoin.
Tollens Reagent Preparation
Since Tollens Reagent has a relatively short shelf life, the reagent is not commercially sold. Therefore, this reagent is often prepared directly in the laboratory. One such method for the preparation of Tollens Reagent is described below:
Step 1
A few drops of dilute NaOH are introduced to an aqueous solution of silver nitrate. The aqueous solution of silver nitrate contain silver aquo complexes wherein water acts as a ligand. The hydroxide ions now convert these silver aquo complexes into silver oxides. This silver oxide (given by Ag2O) precipitates as a brown solid from this solution. The reaction can be written as follows.
2AgNO3 + 2NaOH → Ag2O (brown ppt) + 2NaNO3 + H2O
Step 2
Read more : Which Pillars Of Eternity Is Better
The brown precipitate of silver oxide generated in step 1 is now dissolved with aqueous ammonia. The solution which formed from this addition of aqueous ammonia contains the [Ag(NH3)2]+ complex. This complex is the primary component of Tollens Reagent. The reaction can be written as:
Ag2O (brown ppt) + 4NH3 + 2NaNO3 + H2O → 2[Ag(NH3)2]NO3 + 2NaOH
Tollens Test
When an aldehyde is introduced to the Tollens reagent, two things occur:
The aldehyde is oxidized by the Tollens reagent and forms a carboxylic acid. This reaction can be written as follows:
The silver ions present in the Tollens reagent are reduced into metallic silver. Generally, the Tollens Test is carried out in clean test tubes made of glass. This is because the reduction of the silver ions into metallic silver form a silver mirror on the test tube. This silver mirror is illustrated in the example below. Tollens test is commonly referred to as the Silver Mirror test due to the formation of this layer of metallic silver on the test tube.
Source: https://t-tees.com
Category: WHICH
