The glovebox allows for manipulation of air- and moisture-sensitive reagents and reactions in a similar manner as those performed on the benchtop. This is achieved by maintaining an inert atmosphere in the glovebox (< 1 ppm oxygen and moisture), and the user carrying out manipulations through gloves on the side of the glovebox. The inert gas is typically nitrogen, though argon and even helium may be used. A single glovebox has room for one user, or two gloves. Two users can work side-by-side in a double glovebox, which has four gloves in total. Each glovebox will have slightly different configurations and controls, depending on the manufacturing company. The principles discussed herein can be applied to most standard gloveboxes.
The Main Chamber:
You are viewing: How Fast A Glove Box Without Circulation Increase In Oxygene
The main chamber of the glovebox is comprised of a metal box (usually stainless steel) with polycarbonate windows on one or more sides of the box (Figure 1). Butyl gloves are fitted to the windows, allowing for manipulation inside the glovebox by external users. The box is constructed to be gas-tight, maximizing the integrity of the gas atmosphere within the box. Typically, it is run at a positive pressure, so the gloves stick out of the box. However, when working with extremely toxic or radioactive materials, the box may be run at a negative pressure as to minimize exposure risks.
 Figure 1. The glovebox, showing the main chamber, the gloves, the control panel, and the large/small antechambers. The gloves stick out when it is run at positive pressure.
Figure 1. The glovebox, showing the main chamber, the gloves, the control panel, and the large/small antechambers. The gloves stick out when it is run at positive pressure.
The pressure within is glovebox is generally maintained between ~ 3 and 6 mbar above atmospheric pressure and is regulated via electronics (Figure 2). The user can have additional control by raising or lowering the pressure via a footswitch. The pressure is increased by flowing more gas into the system, and decreased via evacuation from the vacuum pump.
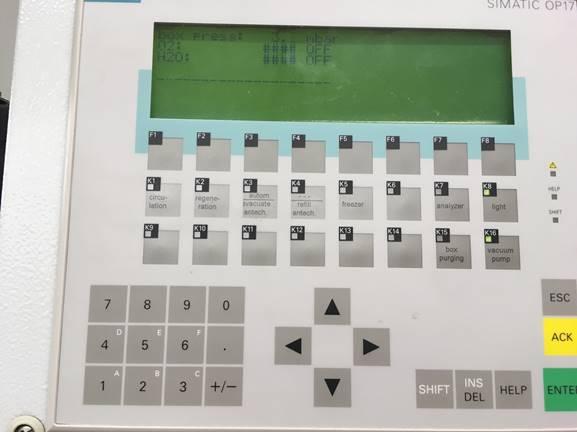 Figure 2. The control panel controls the pressure, circulation, purge, light, regeneration, and the large antechamber.
Figure 2. The control panel controls the pressure, circulation, purge, light, regeneration, and the large antechamber.
Modern boxes are often fit with electrical feedthroughs, and so spectroscopy can be run in the box without having to bring the spectrometer into the box. Coldwells and freezers allow for reactions and storage of chemicals at reduced temperatures, respectively. Gas and vacuum hookups are also possible for the addition of secondary gases to experiments and the removal of solvents from reactions, respectively.
A source of contamination is from the solvents, reagents, and materials brought into the box. If solvents are not properly degassed and dried, then they can add moisture and oxygen to the glovebox atmosphere. Furthermore, they can react with and ruin the catalyst used to keep the atmosphere inert. Likewise, porous materials such as paper must be properly dried and allowed to fully outgas in the antechamber to minimize contamination.
The gloves are a major source of contamination. Because they are porous, air will flow into the glovebox, even when at a positive pressure. The rate of contamination will depend on both the glove material and thickness; typical values for a single glovebox (box with two gloves) are increases in impurities by 59 ppm/h when in use.1 This of course assumes that the atmosphere within the box is not continuously being purified or replaced. Because users generate heat and sweat, the contamination rate can increase to 500 ppm/h when the box is in use. Moreover, holes in the gloves will greatly increase the exchange of air with inert gas within the box.
Read more : How To Act Like Donald Glover
To maintain a good environment, it is thus essential to have a method to keep the atmosphere within the box clean!
The Circulator, Catalyst, and Purging:
To maintain the inert atmosphere, the inert gas (most commonly nitrogen) is circulated from the main chamber to a catalyst, and back to the main chamber. The catalyst (Figure 3) is comprised of molecular sieves and a copper-containing catalyst. The molecular sieves adsorb water and solvent from the gas, while the copper catalyst reacts with oxygen. The two components work together to maintain an inert atmosphere that is free of moisture and oxygen. Large gloveboxes often have a fan in the middle of the chamber to help circulate the gas within the chamber. Gas-flow through the catalyst is done with the circulator. At the inlet and outlet of the chamber are filters, to minimize contamination via small particles.
 Figure 3. The catalyst container is connected to the main chamber by two valves, which allows the atmosphere to circulate through the catalyst bed.
Figure 3. The catalyst container is connected to the main chamber by two valves, which allows the atmosphere to circulate through the catalyst bed.
Over time, the catalyst of the glovebox becomes deactivated (the sieves become saturated with moisture/solvent, and/or the copper catalyst becomes inactive), and needs to be regenerated to maintain low levels or moisture and oxygen. This is done by regenerating the catalyst by heating it under a stream of hydrogen, stabilized by nitrogen (forming gas). The hydrogen serves to remove all solvent and water from the sieves, and to reduce the copper catalyst, that in the process releases water. The water and released solvent are removed via a vacuum pump.
The copper catalyst can be poisoned by certain solvents and volatile chemicals, and thus it is critical to minimize exposure to these impurities. This includes ether solvents, amines (temporary poison), halogens, alcohols, and sulfur-containing compounds (permanent poisons). The presence of the molecular sieves in the catalyst greatly reduces exposure of the copper catalyst to these chemicals, but over time, the copper may become deactivated, and the entire catalyst bed-sieves and copper-must be replaced.
To minimize contamination of the catalyst with undesirable chemicals, the main glovebox chamber can be isolated from the catalyst (by turning off circulation of the atmosphere through the catalyst) when chemicals are being used. The glovebox chamber can be purged (in essence replacing the atmosphere with a fresh supply of gas) before turning the circulation back on. This also ensures that the glovebox atmosphere does not contain trace solvents, which can impact chemical reactions or appear in NMR spectra when samples are prepared inside the glovebox. The length of the purge depends on how much of the inert atmosphere needs to be replaced. For instance, after ~ 5x the volume of the glovebox has been displaced by fresh gas, ~ 1% of the old inert gas remains; this decreases to 0.1% with 7x the volume change.1 The time will be determined by the flow rate of the inert gas into the chamber.
The Antechamber:
Chemicals and supplies are brought in and out of the glovebox via the antechamber (Figure 4). This is a sealed compartment that connects the glovebox to the outside, and can be evacuated with a vacuum pump and re-filled with inert atmosphere. To minimize contamination by air, users will typically run 3 cycles of purge/fill, with the time of evacuation depending on the size of the antechamber as well as what items are to be brought into the glovebox. Items that have a high surface area and are porous must be evacuated for a longer time, to ensure displacement of air.

Figure 4. The large and small antechambers; each have their own pressure gauge and valve.
In general, the fraction of air remaining in the chamber after each cycle of pumping and filling is given by Equation 1,1 where Af = fraction of air remaining, f is the vacuum pressure attainable (in atmospheres), and n is the number of cycles.
Af = fn (1)
Thus, after 2 cycles, a pump that can achieve 1 torr (1.3 x 10-3 atm) will have 1.7 ppm air (in inert gas). This number drops to 2.2 ppb air (in inert gas) after 3 cycles.
The time it takes to evacuate the chamber will depend on the chamber volume and pumping speed. This can be approximated by Equation 2,1 where t is time (min), V is volume of the chamber (L), S is the pumping speed of the vacuum (L/min), and P1 and P2 are the initial and final pressures, respectively.
(2)
While older boxes have manual valves to open and close the vacuum/inert gas inlet to the chambers, modern boxes make use of electronic controls, and the process can even be automated.
Sensors and Controls:
Many modern gloveboxes make use of an electronic display and touchpad to control the gloveboxes (Figure 2). For instance, turning the circulator on and off, purging, opening and shutting valves on the antechamber, etc., are easily applied with the push of a button. The display can also monitor levels of oxygen and moisture in the glovebox, if sensors are installed, which greatly facilitates ensuring an inert environment. However, chemical sensors can likewise be used. Diethylzinc will smoke in the low ppm range of oxygen, and forms a white residue in the presence of water. Sodium benzophenone and the Ti(III) metallocene synthesized in the Schlenk Line module are also common indicators in the glovebox to ensure that the atmosphere is free of moisture and oxygen. Sodium benzophenone can also be used to ensure the removal of moisture from solvent. This purple radical indicator turns blue then colorless in the presence of moisture or oxygen.
Source: https://t-tees.com
Category: HOW
