How do you make PROTACs?
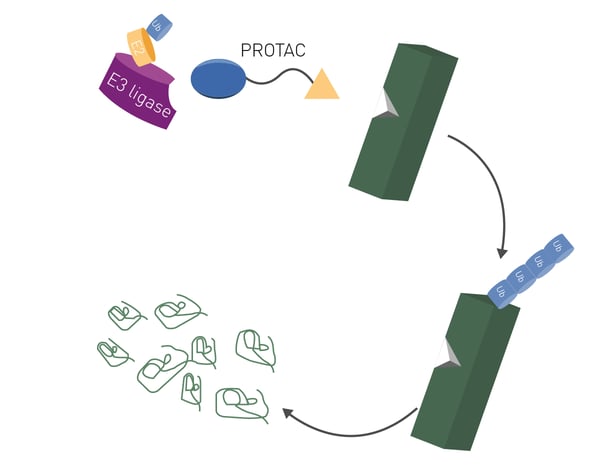
PROteolysis TArgeting Chimeras or PROTACs are an emerging class of molecules that offer new ways to attack disease-causing proteins. They offer the distinct advantage of being able to tackle many previously undruggable targets.1 A PROTAC consists of two separate molecules bound together to form a two-pronged molecular entity (Fig. 1). These two-headed molecules are constructed to selectively degrade disease-causing proteins using the cell’s very own protein disposal machinery, the ubiquitin-proteasome system.
 The concept underpinning PROTACs involves a bifunctional molecule that consists of two essential components: a ligand for the target protein and another ligand for an E3 ubiquitin ligase enzyme. The target ligand binds the protein of interest while the E3 ubiquitin ligase ligand recruits an E3 ligase enzyme (Fig. 1). The recruited ligase brings the target protein into proximity to the ubiquitin-proteasome system, the cell’s very own disposal system responsible for protein degradation.
The concept underpinning PROTACs involves a bifunctional molecule that consists of two essential components: a ligand for the target protein and another ligand for an E3 ubiquitin ligase enzyme. The target ligand binds the protein of interest while the E3 ubiquitin ligase ligand recruits an E3 ligase enzyme (Fig. 1). The recruited ligase brings the target protein into proximity to the ubiquitin-proteasome system, the cell’s very own disposal system responsible for protein degradation.
You are viewing: Which Phrase Describes The Purpose Of Ubiquitin
Once the target protein and E3 ligase are brought together, the ubiquitin ligase adds ubiquitin molecules to the target protein marking it for degradation. Once marked, the tagged protein is recognized by the proteasome and degraded into smaller peptides and amino acids. This degradation process takes the disease-causing protein out of circulation (Fig. 2). 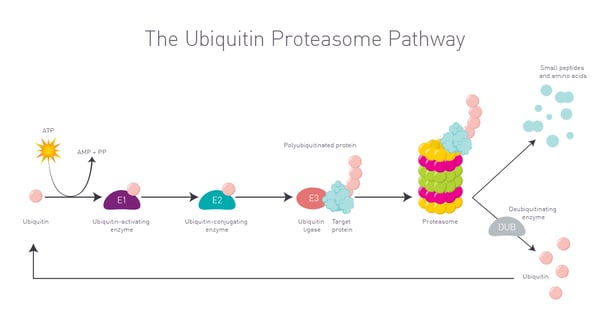 One of the big advantages of PROTACs is their ability to target proteins that were previously classed as undruggable using traditional small molecule inhibitors. Some disease-causing proteins have inaccessible or atypical active sites. In some cases, disease-causing proteins completely lack any enzymatic activity. This limits the design and use of small molecules as drugs. By focusing on protein degradation instead of inhibition, PROTACs offer a new approach to controlling protein levels in the cell. This has the potential to unlock new therapeutic opportunities for a wide range of diseases including cancer, neurodegenerative disorders and autoimmune conditions. The potential for PROTACs as therapeutics is immediately relevant to disease-related non-enzymatic proteins including transcription factors for which small molecule inhibitors have proven to be ineffective.
One of the big advantages of PROTACs is their ability to target proteins that were previously classed as undruggable using traditional small molecule inhibitors. Some disease-causing proteins have inaccessible or atypical active sites. In some cases, disease-causing proteins completely lack any enzymatic activity. This limits the design and use of small molecules as drugs. By focusing on protein degradation instead of inhibition, PROTACs offer a new approach to controlling protein levels in the cell. This has the potential to unlock new therapeutic opportunities for a wide range of diseases including cancer, neurodegenerative disorders and autoimmune conditions. The potential for PROTACs as therapeutics is immediately relevant to disease-related non-enzymatic proteins including transcription factors for which small molecule inhibitors have proven to be ineffective.
The design of proteolysis targeting chimeras involves the careful selection of ligands for the target molecule and the E3 ligase as well as decision making about the linker molecule connecting both functional ligands. Once a target protein is identified, a ligand is chosen that binds to the target protein. The ligand can be designed to target a particular binding pocket or other site on the protein. An E3 ligase enzyme is identified that will transfer ubiquitin molecules to the target protein (ubiquitin ligase). In addition, a ligand is chosen that specifically binds to the E3 ligase. This ligand acts as a recruiter bringing the E3 ligase and the target together.
Read more : Which Of These Diagrams Is A Convex Mirror
The ligands for the E3 ligase and the target protein are connected by a linker molecule. The choice of linker depends on the specific target protein and the desired degradation rate.
Examples of linkers include peptides, small molecules (organic compounds), hydrazones and disulfides. Peptide linkers include Glu-Glu, Lys-Lys and Arg-Arg. Alkyl chains, alkynes and azides are often used as small molecules. Hydrazone linkers are commonly used in PROTACs because of their stability and rapid degradation in cells. Disulfide linkers are stable under physiological conditions but offer the advantage of being able to be cleaved in the presence of reducing agents.
With all the parts selected, the designed PROTAC molecule is then synthesized and tested in cellular and animal model systems to evaluate efficacy and selectivity. Iterative optimization may be performed to improve different properties, including affinity for the target protein and ligase, cell permeability, stability and selectivity. Computational modeling, structure-based drug design and high-throughput screening using microplate readers are often used to aid in the rational design of PROTAC molecules.
From proof of concept to 20 years of advancement
The first proof of concept for PROTACs was reported in a landmark study published in 2001 by Craig Crews and colleagues. The study involved demonstration of the efficacy of PROTAC-1 for the targeted degradation of methionine aminopeptidase-2 (MetAP-2). This groundbreaking study provided the first experimental evidence that small molecule-induced protein degradation using PROTACs was feasible and effective.2 The success of PROTAC-1 has spurred further advancements and optimization of PROTAC design, leading to the development of diverse PROTAC molecules targeting different disease-causing proteins.
PROTACs in action: some examples
Read more : Which Fraction Is Equivalent To 3/5
Many PROTACs have been developed over the past years. ARV-110 is a PROTAC designed to degrade the androgen receptor which is implicated in prostate cancer. It consists of an androgen receptor ligand known as bicalutamide linked to cereblon (CRBN) as the E3 ligase (Fig. 3). 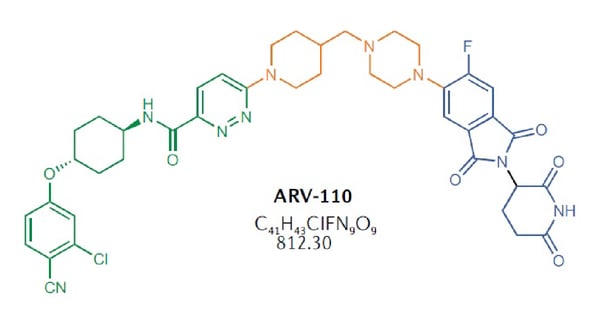 ARV-471 is a PROTAC designed to degrade the estrogen receptor which is involved in certain types of breast cancer. It consists of the receptor ligand fulvestrant linked to cereblon as the E3 ligase. Effective degradation of the estrogen receptor has been demonstrated along with antitumor activity in breast cancer model systems.
ARV-471 is a PROTAC designed to degrade the estrogen receptor which is involved in certain types of breast cancer. It consists of the receptor ligand fulvestrant linked to cereblon as the E3 ligase. Effective degradation of the estrogen receptor has been demonstrated along with antitumor activity in breast cancer model systems.
PROTAC VZ185 degrades the bromodomain protein BRD9 and its close homolog BRD7. BRD9 is a component of the SWI/SNF (SWItch/Sucrose Non-Fermentable) chromatin remodeling complex, a group of proteins that remodel the way DNA is packaged. BRD9 has been implicated in various cancers as a regulator of tumor cell growth. The VZ185 PROTAC consists of a BRD9 ligand linked to an E3 ligase ligand.
Molecular glues, monovalent degraders
The repertoire of targeted protein degradation has expanded in recent years to include new strategies to modulate protein levels. Molecular glues and monovalent degraders are alternative approaches to achieve targeted protein degradation (Fig. 4). Molecular glues are small molecules that bind to the target protein or E3 ligase, bringing them into proximity and promoting the degradation of the target protein. Molecular glues are not bifunctional like PROTACs but consist of a single molecule that induces interaction between the target protein and the E3 ligase.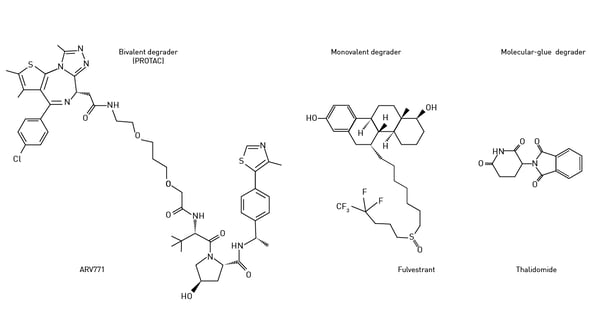 Stuart Schreiber coined the phrase “molecular glue” back in 1992 in the context of immunophilins.3 Cyclosporin and cyclophilin were amongst the first molecules shown to work in this way.
Stuart Schreiber coined the phrase “molecular glue” back in 1992 in the context of immunophilins.3 Cyclosporin and cyclophilin were amongst the first molecules shown to work in this way.
Examples of molecular glues that induce degradation of protein targets are thalidomide, lenalidomide, and pomalidomide (immunomodulatory or IMiDe drugs). Each of these degraders brings the target protein into proximity with cereblon, the E3 ubiquitin ligase mentioned earlier. Thalidomide, a potent teratogen, was initially developed as a sedative and anti-inflammatory agent but was later found to have anti-angiogenic and immunomodulatory effects. Thalidomide leads to protein degradation through its ability to bind the cereblon E3 ligase (Fig.5).  Monovalent degraders are designed with only one binding domain that specifically recognizes the target protein. The monovalent degrader binds to the target protein and recruits the endogenous E3 ligase machinery to induce protein degradation. This approach relies on the natural interaction between the target protein and the E3 ligase.
Monovalent degraders are designed with only one binding domain that specifically recognizes the target protein. The monovalent degrader binds to the target protein and recruits the endogenous E3 ligase machinery to induce protein degradation. This approach relies on the natural interaction between the target protein and the E3 ligase.
Source: https://t-tees.com
Category: WHICH