If you must source disposable gloves for your organization, be it a healthcare facility or production plant, you have many options to consider. You might need to determine whether to order nitrile, vinyl or thermoplastic elastomer (TPE) gloves alongside figuring out the correct quantities. Non-sterile gloves have many applications outside of clinical use, in everything from food processing to chemical handling.
Many in the healthcare sector wonder whether sterile gloves are necessary or if non-sterile gloves will do. Both have their place in medical procedures and patient care. The right choice depends on many factors.
You are viewing: Are Gloves Effective When Handling Ethylene Oxide
What Does Sterile Mean?
Sterilization has a distinct medical definition and must be distinguished from disinfection. Something sterile is free from all microbial life forms. Healthcare facilities can carry out sterilization through physical or chemical methods. In contrast, disinfection eliminates most or all pathogenic microorganisms except for bacterial spores. Medical facilities can disinfect inanimate objects with liquid chemicals or pasteurization.
It’s critical to recognize this distinction. Many in the industry confuse the meaning of sterile or use it interchangeably with disinfection. Some products will be labeled “partially sterile,” which means the item has been disinfected, not sterilized.
It’s also useful to understand that sterility is measured through statistical probability. While governing bodies such as the Food and Drug Administration (FDA) can test for sterility, the testing methods cannot entirely prove sterility. For example, the analysis can introduce microbes onto sterile devices. Instead, sterility is assured if a particular sterilization technique has been applied long enough that the probability of survival is less than one microorganism.
The FDA recognizes many acceptable sterilization techniques. The four methods with the longest history of safe and effective use include:
- Dry heat: Dry heat is the preferred sterilization method when a device might get ruined with steam exposure or is impenetrable to moisture. With this method, items are placed in a dry heat cabinet at high temperatures ranging from 300 to 340 degrees Fahrenheit for up to 150 minutes.
- Ethylene oxide: A colorless, flammable and explosive gas, ethylene oxide can sterilize objects in a fixed, rigid chamber. It’s excellent for heat- and moisture-sensitive materials. The process can take one to six hours, and devices must be aerated after sterilization to remove traces of the harmful gas.
- Moist heat or steam: High-pressure saturated steam is a widely used, dependable sterilization method. Manufacturers or healthcare personnel must expose wrapped medical supplies for at least 30 minutes at a temperature of 250 degrees in a gravity displacement sterilizer. In a prevacuum sterilizer at 270 degrees, the dwell time is reduced to just four minutes.
- Radiation: Medical device manufacturers can expose objects to gamma rays in a shielded room. The process destroys microbial DNA molecules using cobalt 60 gamma rays. It can break down the polymers in some single-use devices while strengthening the polymers in others. It’s often used as an alternative to ethylene oxide.
The two techniques used for sterile gloves include ethylene oxide and gamma rays.
One of the major risks associated with surgical and invasive medical procedures is the introduction of pathogens. In healthcare settings like hospitals, dentist offices and clinics, healthcare professionals must avoid exposing patients to infection-causing microbes. Sterilizing single-use devices and tools that will contact patients is crucial to prevent the spread of infections.

How Do Sterile and Non-Sterile Gloves Differ?
Sterilization is crucial for gloves used in many medical procedures. Still, they’re not always necessary. Both have uses and applications in medical settings.
Sterile gloves have been treated with either ethylene oxide or gamma radiation to eliminate all microbes. They’re used for surgical procedures and come in individually wrapped packing.
What does non-sterile mean regarding medical devices? Non-sterile gloves, also called medical or examination gloves, are also highly regulated by the FDA and need certification to be used in medical settings. Their primary job is to act as a physical barrier for germs. They stop any pathogens on a patient or medical professional from transmitting between individuals. While the manufacturer does not sterilize them, they can be later sterilized at a healthcare facility.
Sterile gloves have a stricter Acceptable Quality Level (AQL) of 1.0 to 1.5 AQL, meaning 1%-1.5% will have pinholes. Non-sterile gloves can have an AQL from 1.5 to 2.5, with up to 2.5% of gloves containing pinholes. Another difference is in the cost. Since sterile gloves undergo timely or complicated sterilization procedures and must meet higher packaging standards, they cost more than non-sterile gloves.
Sterile Glove Applications
Sterile or surgical gloves are considered medically necessary for invasive procedures and operations that contact sterile parts of the body. Some applications include:
- Performing catheter insertion and care.
- Inserting central intravenous or arterial lines and performing associated care.
- Changing sterile dressings for surgical wounds.
- Protecting immunocompromised individuals who become heavily compromised and wear non-sterile gloves under normal circumstances.
- Dressing skin or mucous membrane wounds when there’s a high risk of contamination or infection.
- Performing tracheotomies in neonates, premature infants and patients with recurring respiratory infections or while the stoma is healing.
- Delivering babies.
- Handling surgery equipment.
- Handling dialysis catheters or performing dialysis.
Non-Sterile Glove Applications
Read more : What To Say When Someone Dies Condolences In Islam
Clean or non-sterile gloves are acceptable for scenarios such as:
- Dressing a wound healing by secondary intention, such as a wound over six weeks old, pressure sores, leg ulcers, simple grazes and dehisced wounds.
- Removing drains or sutures.
- Performing tracheotomies in a home setting.
- Directly contacting blood, body fluids, mucus membranes and non-intact skin.
- Performing gastrostomies and colostomies.
- Removing urinary catheters or emptying catheter drainage bags.
- Inserting or removing a peripheral intravenous line in a smaller vein.
What Is the Aseptic Technique?
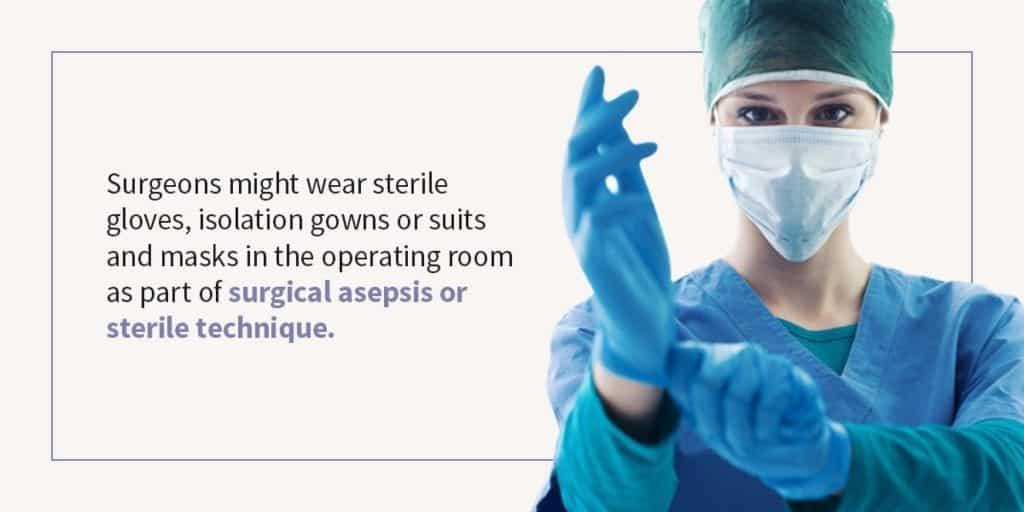
Asepsis, or the aseptic technique, is another term related to sterilization. It involves the exclusion of microorganisms from a medical environment or procedure. Surgeons might wear sterile gloves, isolation gowns or suits and masks in the operating room as part of surgical asepsis or sterile technique. The aseptic technique may involve sterilizing the skin with alcohol and often includes other steps to preserve a sterile environment.
The aseptic technique is critical because of the prevalence of healthcare-acquired infections (HAIs). HAIs are infections patients contract through exposures that occur in the healthcare environment. While one in 31 hospital patients has an HAI at any given time, most are preventable. Some of the most common HAIs include:
- Catheter-associated urinary tract infections.
- Central line-associated bloodstream infections.
- Surgical site infections.
- Ventilator-associated pneumonia.
- Clostridium difficile infections.
The procedures most likely to result in these infections demand an aseptic technique. Healthcare professionals employ it when they are:
- Dressing surgical wounds and burns.
- Performing biopsies.
- Suturing wounds.
- Inserting a urinary catheter, chest tube, intravenous line or wound drain.
- Administering injections.
- Performing surgical procedures.
- Conducting vaginal examinations with instruments.
- Delivering babies.
Healthcare professionals must perform four types of aseptic techniques, including:
1. Barriers
Barrier medical devices stop germs from transferring between healthcare workers, patients and surfaces. Aseptic barriers include:
- Sterile masks
- Sterile gowns
- Sterile drapes
- Sterile gloves
- Protective packaging on sterilized instruments
Before donning sterile barriers, healthcare workers first perform hand hygiene, usually through washing or sanitizing their hands.
2. Equipment and Patient Preparation
Any procedure involving an aseptic technique requires a healthcare professional to prepare both the patient and equipment first. This type of preparation might involve:
- Using antiseptic wipes to disinfect a patient’s skin.
- Sterilizing equipment and instruments.
- Keeping sterilized equipment inside plastic wrappers to prevent contamination before use.
3. Environmental Controls
It’s also critical to consider the patient’s surroundings, or the designated aseptic field. The room where the procedure takes place usually marks the boundary of the aseptic field. Maintaining the aseptic field involves:
- Keeping doors closed.
- Minimizing entries and exits from the defined aseptic area.
- Allowing only one patient per aseptic field.
- Permitting only necessary personnel.
4. Contact Guidelines
Once a healthcare professional has initiated aseptic technique by washing their hands and donning sterile barriers, they must follow certain rules for contact. They may only touch sterile surfaces and instruments in keeping with sterile-to-sterile contact guidelines. They cannot touch any non-sterile surfaces. The sterile devices must follow the same rules. Any sterilized device that falls to the floor, becomes compromised or sustains damage to its wrapper must be removed and resterilized before use.
The Differences Between Aseptic, Sterile and Clean Techniques
It’s easy to misunderstand the relationship between the aseptic and sterile techniques because the two terms go hand in hand. Essentially, sterile describes an object that has been completely rid of microbes. Asepsis describes the conditions required to keep that object sterile. The sterile technique involves methods such as dry heat, ethylene oxide or gamma rays to produce a sterilized device. The aseptic technique involves creating barriers, preparing patients and equipment, controlling the environment and following contact guidelines.
Aseptic technique and clean technique are similar in that they are used during medical examinations and procedures to limit harmful pathogens. When achieving medical asepsis, the goal is to prevent the exposure of any microbes through the use of sterile barriers and materials. When employing a clean technique, the goal is to lower the number of pathogens, or harmful microbes, exposed to the patient.
The clean technique involves three of the same methods as the aseptic one:
- Barriers
- Patient and equipment preparation
- Environmental controls
Besides the absence of sterile-to-sterile contact guidelines, the three methods are applied more loosely in the clean technique. For example, with barriers, the healthcare professional need only use appropriate hand hygiene and non-sterile gloves. Rather than sterilizing tools, the worker ensures they don’t become directly contaminated. The patient also doesn’t need to remain in an aseptic field. Instead, the environment undergoes routine cleaning.
Read more : When Do Lantanas Come Back
Healthcare workers use clean techniques while:
- Giving an injection.
- Emptying a urinary catheter drainage bag.
- Inserting or removing a peripheral intravenous line.
- Removing a urinary catheter.
- Giving a bed bath.

How Does the FDA Verify Sterile Products?
The FDA has rigorous approval processes for medical devices of any kind. When a product is sold and labeled as sterile, it demands more documentation and testing. Different rules apply to products subject to each sterilization technique. Sterile surgical gloves can be safely sterilized using either ethylene oxide or radiation and will be subject to corresponding FDA approval processes.
Gloves Sterilized Through Ethylene Oxide
Manufacturers must submit detailed records and documentation of the sterilization and sterility testing process. The submission should include:
- Description of the sterilizer: This includes information about the ethylene oxide and the site where it is administered. Since gloves treated with ethylene oxide must be aerated to reduce the residual chemicals, manufacturers also describe how the gloves are aerated.
- Cycle parameters: Besides basic information about the process, the documents must include all the parameters throughout each stage of sterilization. They must list the gas concentration, exposure time and temperature, vacuum and gas pressure cycles and other conditions. Essentially, the records must show the process follows validated parameters.
- Microbiological methods: Next, the documents outline the microbial testing methods. They’ll list the growth medium used, incubation temperature and time interval. Using proven testing methods, the manufacturer must demonstrate that sterilized product samples do not grow microbiological colonies.
- Monitoring stability: The manufacturer must have a documented program for tracking the packaging and container closure system’s integrity. The packaging must remain stable through the product’s claimed shelf life.
Gloves Sterilized Through Radiation
Likewise, manufacturers sterilizing their gloves through gamma ray radiation must submit information on:
- Facility and process: When manufacturers use radiation, they must indicate the facility used, source, method of exposure and information about the monitoring devices.
- Product packaging: Manufacturers should also describe the inner packaging and shipping packaging.
- Multiple-dose mapping studies: The radiation process should be documented to identify low and high dose sites and demonstrate the method’s uniformity and replicability.
- Microbiological methods and controls: Next, the documentation will lay out the methods used for microbiological sterility testing and process validation.
- Monitoring stability: As with ethylene oxide sterilization, the manufacturer must document a program for monitoring the packaging’s integrity through the sterile product’s claimed shelf life.
Are Non-Sterile Gloves Still Safe?
While non-sterile gloves aren’t specifically treated to remove all biological contaminants, they can be just as safe for most minor medical procedures. They’re used in clean techniques, which means healthcare professionals follow standard infection control procedures and keep the gloves clean while in use. They wash their hands or use hand sanitizer gel before donning them and avoid touching contaminated surfaces. Non-sterile gloves are perfectly safe for minor medical procedures and have many uses in clinical settings.
A recent study compared non-sterile and sterile glove use in minor skin and dental procedures. The results showed equal rates of postoperative surgical site infections, with a 0.1% difference. When used under the right circumstances, non-sterile gloves are as safe as sterile ones. They still act as a barrier between the healthcare professional and patient, increasing safety.
The difference in safety comes during procedures that put patients at higher risk for HAIs, such as inserting catheters. Sterile gloves are medically necessary when medical procedures and surgeries will expose sterile parts of the body.
What to Consider When Choosing Between Sterile and Non-Sterile Gloves
Healthcare professionals must wear gloves in any scenario where pathogens could pass between themselves and their patients. Certain clinical scenarios require aseptic conditions and the use of sterile gloves. If your facility uses gloves in an aseptic environment for invasive medical procedures or any situation that demands sterility, choose sterile gloves.
In other medical uses, it’s safe to use approved examination gloves or sterile surgical gloves. Whether either type of glove is safe for a particular medical procedure comes down to your facility’s policies and the costs. Facilities usually save the more expensive sterile gloves for when they are medically necessary.
It’s crucial to accurately estimate the supply of sterile gloves needed. Many surgeons double-glove during operations, using two different-colored sterile gloves. This practice protects the inner glove from damage and lets healthcare professionals know if they become compromised during the procedure. If your healthcare professionals use a double-gloving technique, make sure your sterile glove purchases reflect this.
Using non-sterile gloves for any procedure where it is safe to do so will save your organization money. It can also be critical during times of medical supply shortages. If your facility finds sterile gloves in short supply, it’s best to use non-sterile ones where they are safe and approved.
Non-sterile gloves also have many uses outside healthcare facilities and clinical settings. For example, vinyl non-sterile gloves are recommended when working with cleaning detergents and chemicals. They have applications for janitorial work, hairdressing and cosmetology, chemical experiments and food processing. Latex-free, non-sterile TPE gloves are also useful for hairdressing, cosmetology and food handling.
Order Bulk Nitrile Gloves From SUNLINE Supply
Non-sterile nitrile gloves have a broad range of medical uses. They are a latex-free material with a similar feel and superior strength and comfort. The material is more resistant to chemicals, oils and acids than natural rubber and offers three times more puncture resistance than latex. They’re recommended for many medical procedures, especially when the patient or healthcare professional has a latex allergy. They also protect workers exposed to chemicals, pesticides, petroleum and commercial cleaning agents.
At SUNLINE Supply, we work with a wide selection of thoroughly vetted nitrile glove manufacturers to ensure a steady supply of products you can trust. We only use the highest quality vendors. We offer fast delivery and competitive prices. Learn more about our nitrile gloves, and shop our PPE products online today!

Want to Learn More? Check out These Additional Posts
Source: https://t-tees.com
Category: WHEN
