What is the True Solution?
A true solution is one that comprises all the particles in the correct composition and has been properly dissolved. As a result, a solution is known as a real solution.
Table of Contents
-
- Introduction
- Properties of True Solution
- Difference between True Solutions and Colloidal Solutions
- Preparation of True Solution
- Frequently Asked Questions-FAQs
Introduction
A true solution is a homogeneous mixture with consistent properties. Filtration cannot separate the solute from the solution in a true solution. The solute’s particle size is around the same as the solvent’s, and the solvent and solute move through the filter paper together.
You are viewing: What Is The True Solution To The Equation Below
A true solution is a homogeneous mixture of two or more substances in which the particle size of the material dissolved (solute) in the solvent is less than 10-9 m or 1 nm. True solution is exemplified by a simple sugar solution in water.
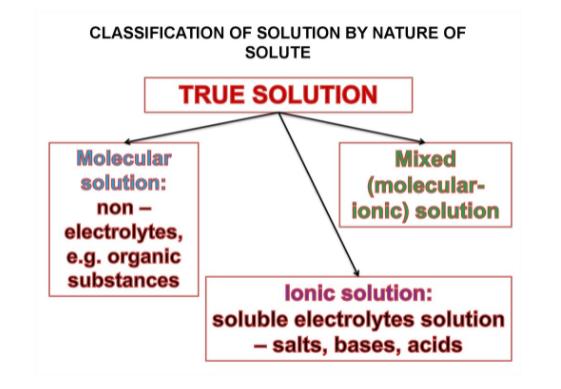
Properties of True Solution
Read more : What Is A Quarter Of Weed
A true solution is a mixture of solute and solvent that is homogeneous. Filtration cannot separate the solute from the solution in a true solution. The solute’s particle size is around the same as the solvent’s, and the solvent and solute move through the filter paper together.
Solute particles are smaller than 1 nm (1 nm =10-9m). The elements do not disperse light and do not exhibit the Tyndall effect. Filtration would not be able to isolate the particles. The outcome is safe (remains uniform). The solution is visible. In a true solution, the solute particles do not settle. Light should not scatter in a true solution. A true solution is transparent and clear.
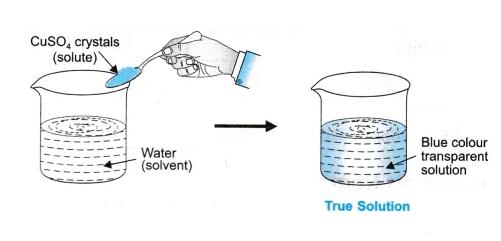
Components of the solution are the substances that make up a homogeneous solution. It consists of two main components: a solvent and a solute. Solvent: A solvent is a component of a solution that dissolves the other components in itself. The majority of the solution is made up of a solvent.
Difference between True Solutions and Colloidal Solutions
In terms of chemistry, Solutions are classified as mixtures of two or more substances in which the solvent is liquid and the solute is liquid, solid, or gas. There are several types of solutions, each with its own set of characteristics, but they can be divided into: True solutions and Colloidal solutions.
Read more : What To Serve With Jamaican Jerk Chicken
The comparison chart is given below.
Basis of Comparison True solution Colloidal solution Definition A true solution is a homogeneous mixture of two or more substances in which the particle size of the material dissolved (solute) in the solvent is less than 10-9 m or 1 nm. A colloid is a mixture in which one material is suspended in another by microscopically scattered insoluble particles. Examples Sugar solution in water. Starch dissolved in water. Nature of the solutions Colloidal solution and suspension are heterogeneous mixtures of two or more substances, whereas the true solution is a homogeneous mixture. Another distinction between these three types of solutions is that True is transparent, while Colloidal is translucent, and Suspension is opaque. A colloidal solution, also known as a colloidal suspension, is a mixture in which the substances are dissolved in a fluid in a normal pattern. A colloid is a very small particle that is evenly distributed in another substance. In nature, these are heterogeneous. External appearance A true solution is one that is both straightforward and transparent. Light should not scatter in a real solution. Filtration cannot distinguish the components of a true solution. The Tyndall effect, which is the scattering of light by particles in a colloid, causes certain colloids to be translucent. Sedimentation Will, not sediment. There will be no sedimentation of particles or colloids. Visibility of particles True Solution particles are invisible to the naked eye. As light travels through colloidal solutions, it is dispersed, with the highest scattered intensity in the plane perpendicular to the light direction. The path beam is clear now. Brownian Movements Brownian motion is observed in true solution particles. Brownian motion is observed in colloidal solution particles.
Preparation of True Solution
1. True solution of common salt:
Pour 100 mL of distilled water into a clean and dry beaker, then add dry common salt. Using a glass rod, stir the contents. To form a true solution, common salt dissolves absolutely.
2. True solution of sugar:
Pour 100 mL of distilled water into a clean and dry beaker, add a few sugar crystals, and stir the contents with a glass rod. To form a true solution, the sugar dissolves in water.
3. True solution of alum:
Pour 100 mL of distilled water into a clean and dry beaker, then add a pinch of alum powder and mix with a glass rod. A true solution is formed when the alum dissolves in water.
Recommended Videos
Types Of Solutions
Source: https://t-tees.com
Category: WHAT
