In general, the Bohr model encapsulates the modern understanding of the atom. This model is frequently depicted with a central atomic nucleus and oval lines representing electron orbits.
The current model of the atom is the “quantum mechanical model” or the “electron cloud model”, which was developed in the 1920s and early 1930s by a number of scientists, including Erwin Schrödinger and Werner Heisenberg. This model is based on quantum mechanics principles, which describe the behaviour of matter and energy at the subatomic level.
You are viewing: Which Best Describes The Current Model Of The Atom
The concept of the atom was proposed by the Greek philosopher Democritus. He stated that all matter is composed of indivisible particles known as atoms that are surrounded by empty space. There were some other theories too before our modern concept of the atom was developed in the nineteenth and twentieth centuries.
In this article, we will study in detail the history of Atomic Models that led to the discovery of the current model of the atom.
1. Atomic Model

Many different atomic models have existed throughout the history of atomic physics. Although the existence of atoms has long been recognized. The four fundamental atomic models are:
- Dalton’s Billiard Ball Model
- Thomson’s Atomic Model or The Plum Pudding Model
- Rutherford’s Planetary model
- Bohr’s Atomic model
1.1 Dalton’s Billiard Ball Model
Dalton’s Billiard Ball Model, proposed by John Dalton in 1803, is an early atomic theory that describes the behavior of matter as being composed of small, indivisible particles called atoms.
In this model, atoms are like billiard balls, which are thought to be solid and indestructible. They are in constant motion and collide with one another, causing changes in their motion and resulting in chemical reactions.
However, Dalton’s model did not explain the internal structure of atoms, and it was later replaced by more advanced theories that provided a more detailed understanding of atomic structure and behavior.
1.1.1 Demerits of Dalton’s Atomic Theory
There are several demerits or limitations of Dalton’s atomic model:
- It did not explain the internal structure of atoms, which was later revealed by more advanced theories such as the atomic models of Niels Bohr and Erwin Schrödinger.
- The model did not account for the existence of isotopes- that are atoms of the same element but they have a different number of neutrons in their nuclei.
- The model did not explain the phenomenon of atomic spectra, which was observed in light emitted or absorbed by atoms.
- The model did not account for the existence of subatomic particles- electrons, protons, and neutrons, which were later discovered by scientists such as J.J. Thomson and Ernest Rutherford.
- The model assumed that atoms were indestructible, which was later disproved by the discovery of nuclear reactions, which can break atoms apart or merge them together to create new elements.
- Dalton’s atomic theory also did not have any concept of chemical bonding, which is the way atoms combine to form molecules.
- All of these limitations and errors in Dalton’s atomic model were later addressed by more advanced theories, such as quantum mechanics and the electron cloud model, which provided a more detailed and accurate understanding of atomic structure and behavior.
1.2 J. J. Thomson’s Model
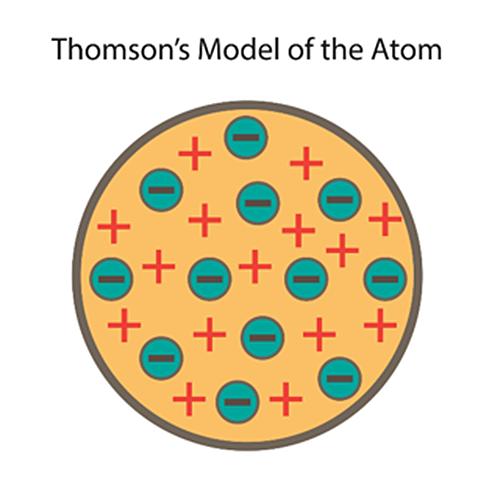
J.J. Thomson’s atomic model, also known as the “plum pudding” model, was proposed in 1904. In this model, Thomson proposed that atoms were composed of negatively charged electrons embedded in a positively charged “soup” or “pudding”.
He had discovered the electron, a subatomic particle while conducting experiments on cathode rays. He proposed that atoms were neutral because the negative charges of the electrons were balanced out by an equal amount of positive charge in the soup.
Thomson’s model was able to explain some previously unexplained phenomena, such as the fact that atoms are electrically neutral, and that the ratio of the charge of an electron to its mass is the same for all elements.
1.2.1 Limitations of J.J Thomson’s Model:
- It could not explain the stability of atoms, as the negatively charged electrons should have been attracted to the positively charged soup and collapsed into the center.
- It could not explain the observed phenomena of atomic spectra.
- It could not explain the phenomenon of radioactivity.
- The model could not explain the existence of isotopes, which are atoms of the same element that have a different number of neutrons in their nuclei.
- The model did not account for the fact that electrons have a wave-like nature, which was later revealed by quantum mechanics.
- The model also did not account for the existence of protons, which were later discovered by Ernest Rutherford and were found to be located in the atomic nucleus.
Read more : Which Is Characteristic Of Low Head Dams
Thomson’s atomic model was later refined and replaced by Rutherford’s planetary model, which proposed that most of the mass and positive charge of the atom is concentrated in the nucleus, while the electrons orbit around it.
1.3 Rutherford Model
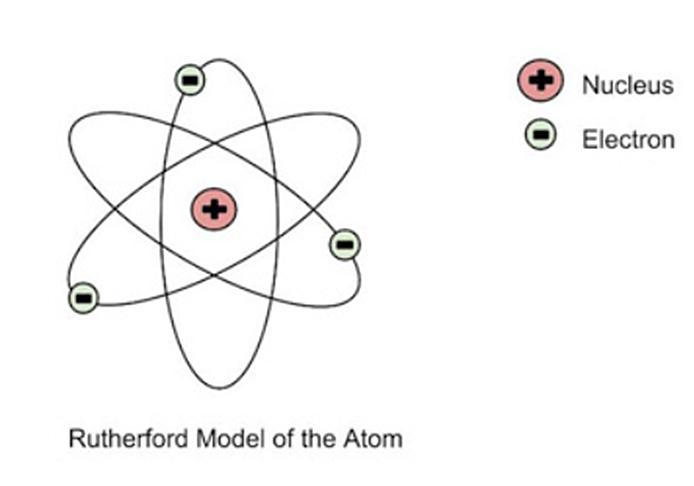
Ernest Rutherford’s atomic model, also known as the planetary model of the atom, was proposed in 1911 based on the results of his famous gold foil experiment. In this experiment, Rutherford and his team fired alpha particles (positively charged particles) at a thin sheet of gold foil. They observed that most of the alpha particles passed straight through the foil, but a small number of them were deflected at large angles.
From these observations, Rutherford proposed that atoms were composed of a small, dense, positively charged nucleus at the center, surrounded by negatively charged electrons that orbited around it like planets around the sun. The nucleus contains protons and neutrons, and the number of protons in the nucleus determines the element.
Rutherford’s model was able to explain several previously unexplained phenomena, such as the stability of atoms, the existence of isotopes, and the phenomenon of radioactivity.
1.3.1 Limitations of the Rutherford Model
- It did not explain the observed phenomena of atomic spectra.
- It did not account for the wave-like nature of electrons.
- It also did not account for the fact that electrons can move in multiple orbits around the nucleus, not only one like the planetary model proposed.
- The model could not explain the stability of atoms. Rutherford’s model failed to explain why the negatively charged electrons do not spiral into the positively charged nucleus.
- The model could not explain the phenomenon of electron spin, which was later discovered by physicists.
- The model did not account for the fact that electrons can be in multiple states at the same time, this is called superposition, which was later revealed by quantum mechanics.
Rutherford’s model was later refined and replaced by the quantum mechanical models such as the wave-mechanical model of Schrödinger and Heisenberg, which provided a more detailed and accurate understanding of atomic structure and behavior.
1.4 Bohr’s Model
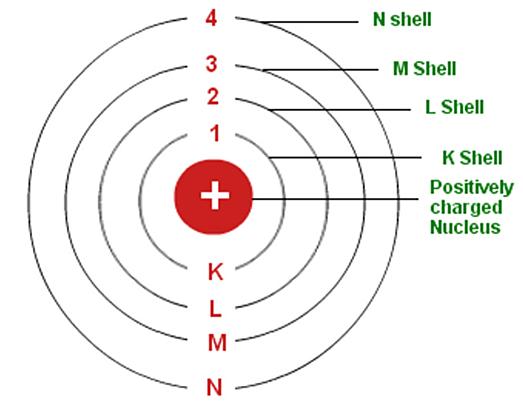
Niels Bohr’s atomic model, also known as the Bohr model, was proposed in 1913 as a refinement of Rutherford’s planetary model. In the Bohr model, Bohr proposed that electrons orbit the nucleus in specific, discrete orbits, or “energy levels”, and that the energy of an electron is determined by its orbit.
- Bohr proposed that electrons can only exist in certain energy levels and that when an electron jumps from one energy level to another, it must either absorb or emit a photon of light. This explained the phenomenon of atomic spectra, where atoms emit or absorb light at specific wavelengths.
- Bohr’s model also introduced the concept of the quantization of angular momentum, which is the product of the mass of the electron, its speed, and the distance of the orbit from the nucleus.
- Bohr’s model was able to explain several previously unexplained phenomena, such as the stability of atoms and the phenomenon of atomic spectra.
1.4.1 Limitations of Bohr’s Model
- It only worked for simple atoms such as hydrogen, and could not be applied to more complex atoms.
- It could not explain the wave-like nature of electrons, which was later revealed by quantum mechanics.
- It did not account for the phenomenon of electron spin, which was later discovered by physicists.
- It did not account for the fact that electrons can be in multiple states at the same time, this is called superposition, which was later revealed by quantum mechanics.
- The model also failed to explain the phenomenon of electron-electron interactions in multi-electron atoms, which is important for chemical bonding.
- It did not account for the fact that electrons can be in multiple states at the same time, this is called superposition, which was later revealed by quantum mechanics.
- It also could not explain the phenomenon of the fine structure of spectra, the splitting of spectral lines into multiple components, which was later explained by the relativistic effects on the electron’s motion.
Bohr’s model was later refined and replaced by the wave-mechanical model of Schrödinger and Heisenberg, which provided a more detailed and accurate understanding of atomic structure and behavior.
2. Current Model of the Atom
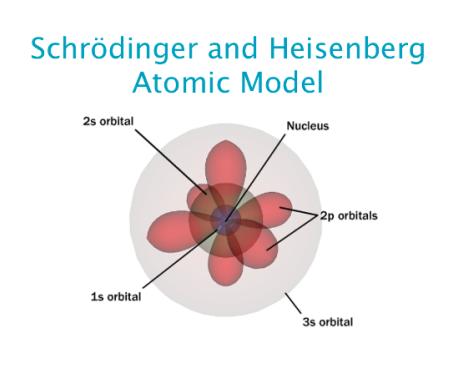
Erwin Schrodinger developed an atomic model in 1926 that took into account the electron’s wave and particle nature. This is known as the atomic quantum mechanical model.
It describes the electron as a three-dimensional wave in the positively charged nucleus’ electronic field. In this field, the wave motion of the electron is described using a differential equation known as the Schrodinger wave equation.
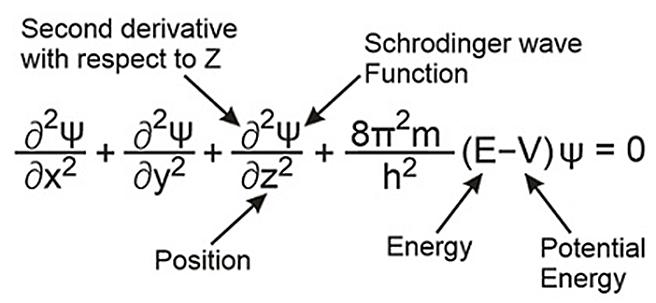
Here, x, y, and z are the space coordinates.
Read more : Which Of The Following Is True About Histone Acetyltransferase Hat
M= Mass of the electron
E= Total energy of the electrons
V = Total potential energy of the electrons
ψ (Psi)= Wave function of the electron.
2.1 Characteristics of Quantum Mechanical Model
The Schrodinger wave equation depicts the atom more accurately than Bohr’s model. Some of the most important aspects of an atom’s Quantum Mechanical Model are as follows:
- The energy of electrons in an atom is quantized, which means that it can only have certain specific values. The solutions to the Schrodinger wave equation generate the quantized energy levels in which electrons can exist.
- An electron’s exact position and velocity (or momentum) cannot be determined at the same time. As a result, the electron’s path is only probable and not exact. This eventually resulted in the concept of atomic orbitals.
- The wave function ψ, also known as the orbital wave function, represents the atomic orbital. Since an electron can have a variety of such wave functions, the corresponding atomic orbitals are also possible. Each orbital of the electron has a specific amount of energy, and it can only have two electrons.
- The probability of finding an electron at a point in an atom is proportional to the square of the wave function i.e., [ψ]2. It is positive and is also known as a probability density. The value of [ψ]2 at various points within the atom can be used to predict the region around the nucleus where the electron will most likely be found or located.
Key Takeaways
- The atomic model has evolved through various stages, with various understandings of the structure and composition of the atom.
- Democritus, the Greek philosopher, believed that all matter was made up of the same small objects known as atoms.
- Dalton’s model proposed that chemical reactions were the result of atom re-arrangements in the object.
- Successive atomic models, such as those proposed by Thomson and Rutherford, altered our understanding of the atom’s charge because they included electrical charges and described how they were distributed within the atom.
- The most widely accepted atomic model today is the quantum mechanical model.
- Bohr’s model and the quantum atomic model altered our understanding of the nature of the atom and how electrons interact within it. Electrons in Bohr’s model move between orbits based on their energy levels. The quantum model introduced uncertainties in the sense that electrons are understood to move in defined areas without us being able to locate their position beyond the probability of their existence in a certain position.
FAQs
What is the current model of the atom?
The current model of the atom is known as the “quantum mechanical model” or the “electron cloud model.” It describes the atom as a small, dense nucleus containing protons and neutrons, surrounded by a cloud of electrons that occupy energy levels or “shells.”
When was the first atomic model proposed?
The first atomic model was proposed in 1803 by John Dalton. He proposed that atoms were indivisible and indestructible particles that make up all matter.
Who proposed the plum pudding atom model?
In 1904, J.J. Thomson proposed the plum pudding model of the atom. He proposed that atoms were composed of a uniform distribution of positive charge with negatively charged electrons embedded in it, similar to raisins in a pudding.
Who proposed the planetary model of the atom?
In 1913 the planetary model of the atom was proposed by Niels Bohr. He proposed that electrons orbit around the nucleus in specific energy levels or “shells,” similar to planets orbiting the sun.
What did the Rutherford model propose about the structure of the atom?
The Rutherford model proposed that atoms have a small, dense nucleus containing protons (positively charged and neutrons (neutral), surrounded by a cloud of negatively charged electrons. The electrons were thought to orbit the nucleus like planets orbit the sun.
Source: https://t-tees.com
Category: WHICH
