The Photoelectric Effect
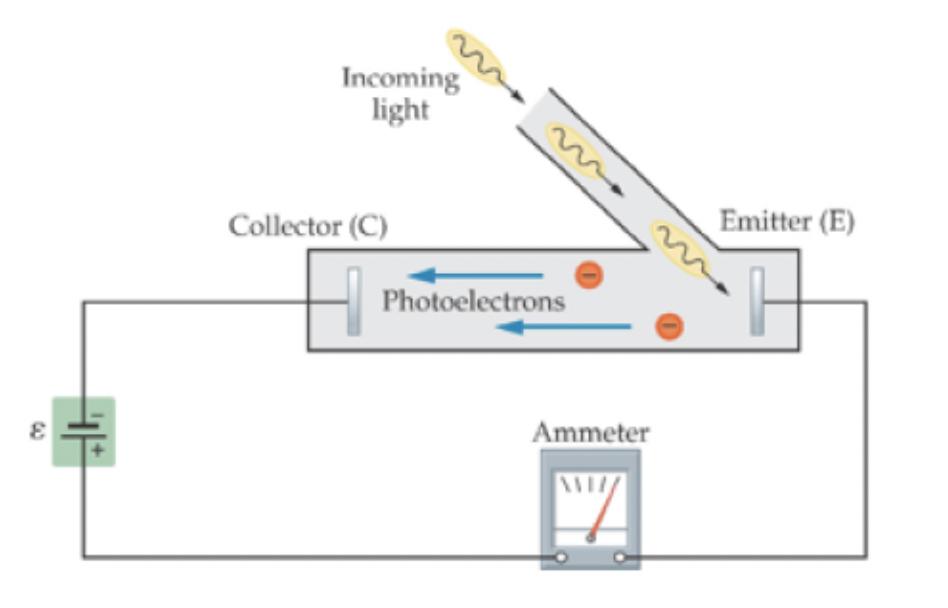
One of the first experiments which is exhibited strong experimental evidence of light behaving like a particle was the photoelectric effect, which first led Albert Einstein to develop the particle model of light. In the photoelectric effect, a beam of incoming light shines on a metallic surface. When the beam hits the metal, it eject electrons from the metal and sends the electrons down a tube to a collector. To do so, the light must provide the electrons with enough energy to break their bonds to the metal, and sufficient kinetic energy to reach the collector. Reaching the collector requires a certain amount of minimum kinetic energy at emission, because an electric field exists between the collector and the emitter that acts to slow down the electrons on their path. The basic set-up is shown in the figure below.
Figure 9.2.1: The Experimental Setup for the Photoelectric Effect
You are viewing: Which Model Of Light Helps Explain The Photoelectric Effect
Read more : Which Statement Accurately Describes A Data Governance Team
The photoelectric experiment allows us to test the wave model against the particle model, for this particular setup. As an experimenter, we have control over both the intensity of the light and the frequency of the light. We can independently vary one or the other, and note the effect, enabling us to determine the appropriate model for this system.
The photoelectric effect can be explained using the conservation of energy. Light brings in a certain amount of energy. If the energy is sufficiently high, it frees an electron from the metal. Different metals bind the electrons with different amounts of energy. If the incident light has less energy than the this binding energy, the electrons remain attached to the plate. In addition, the experiment was able to measure the amount of energy that the ejected electrons had.
Scientists who carried out these experiments observed the following properties:
- Higher intensity beams free more electrons.
- Higher frequency beams result in electrons with higher speeds.
- Changing the beam intensity has no effect on electron speed.
- Changing the frequency of the beam has no effect on the number of electrons freed (provided the frequency is high enough that some electrons are freed).
These results all support the particle model of light for the following reasons. It was postulated that beams with higher intensities contain more quanta of light, known as photons. As a result higher intensity beams free more electrons, because more photons are present to transfer energy. However, the amount of energy one photon can transfer to an electron is determined by the photon’s frequency. Increasing the frequency of incoming light increases the energy transferred to the electrons, which is why higher frequency beams produce electrons with more kinetic energy. Thus, it was postulated that the energy of an individual photon depends on its frequency the following way:
Read more : The Truth Behind Personal Troubles and Public Issues
[E_{text{photon}} = h f=dfrac{hc}{lambda}label{Ephoton}]
where (h= 6.636 times 10^{-34} text{ J s}) is Planck’s constant, and (c=3.0times 10^8 m/s) is the speed of light in a vacuum.Frequency is what determines the type of light we’re discussing, whether it different colors in the visible range, a radio wave, or an X-ray. The photons of different colors or different types of light have different frequencies and therefore have different energies. At a particular frequency, one photon is the smallest amount of light that can exist.
To consider the implications of the particle model, it is helpful to think about monochromatic light, many photons all with the same frequency, like light produced by a laser. We consider two properties of the light: intensity (i.e. brightness) and the amount of energy the light is able to transfer into another system, like an electron orbiting a nucleus. Returning to the photoelectric effect, compare two beams of light with equal intensity, but different frequencies. From our relationship for the energy of a photon in Equation ref{Ephoton}, we conclude that the beam with the higher frequency has photons with higher energy. Thus, the high frequency beam is capable of transferring larger amounts energy into another system. But if the intensities of the beams are same, the total energy transferred by each beam is the same. This tells us that the beam with the higher frequency has fewer photons. But in the wave model, the same intensity of each beam means they must have the same amplitude. The energy in a wave is related to its amplitude, so it would seem both light beams must have equal ability to transfer energy. Clearly, the two models lead to different hypotheses.
Next, consider the action of increasing the beam’s intensity. In the particle model, we would describe this as adding more photons to the beam, but each particular photon still only carries a certain amount of energy. Thus, more electrons are ejected, but each ejected electron still has the same kinetic energy. Using the particle model, we conclude that the brightness of the beam does not influence how much energy any particular photon can transfer to another system. In the wave model, a greater brightness would indicate a larger amplitude wave; we would conclude that greater intensity waves have the ability to transfer larger amounts of energy into another system. Again, the models make different predictions.
Source: https://t-tees.com
Category: WHICH