Radioactivity and Nuclear Chemistry
3.1 Major Forms of Radioactivity
Alpha Particle (α)
Beta Particle (β)
Gamma Radiation (γ)
Positron Emission (β+ decay) and Electron Capture
Nuclear Fission
3.2 Radioactive Half Lives
3.3 Biological Effects of Radiation Exposure
3.4 Uses of Radioactive Isotopes
3.5 Chapter Summary
3.6 References
Radioactivity and Nuclear Chemistry
Atomic theory in the nineteenth century presumed that nuclei had fixed compositions. But in 1896, the French scientist Henri Becquerel found that a uranium compound placed near a photographic plate made an image on the plate, even if the compound was wrapped in black cloth. He reasoned that the uranium compound was emitting some kind of radiation that passed through the cloth to expose the photographic plate. Further investigations showed that the radiation was a combination of particles and electromagnetic rays, with its ultimate source being the atomic nucleus. These emanations were ultimately called, collectively, radioactivity.
Following the somewhat serendipitous discovery of radioactivity by Becquerel, many prominent scientists began to investigate this new, intriguing phenomenon. Among them were Marie Curie (the first woman to win a Nobel Prize, and the only person to win two Nobel Prizes in different sciences—chemistry and physics), who was the first to coin the term “radioactivity,” and Ernest Rutherford (of gold foil experiment fame), who investigated and named three of the most common types of radiation. During the beginning of the twentieth century, many radioactive substances were discovered, the properties of radiation were investigated and quantified, and a solid understanding of radiation and nuclear decay was developed.
You are viewing: Which Phrase Describes One Characteristic Of Radioactive Elements
The spontaneous change of an unstable nuclide into another is radioactive decay. The unstable nuclide is called the parent nuclide; the nuclide that results from the decay is known as the daughter nuclide. The daughter nuclide may be stable, or it may decay itself. The radiation produced during radioactive decay is such that the daughter nuclide lies closer to the band of stability than the parent nuclide, so the location of a nuclide relative to the band of stability can serve as a guide to the kind of decay it will undergo (Figure 3.1).
Figure 3.1 A nucleus of uranium-238 (the parent nuclide) undergoes α decay to form thorium-234 (the daughter nuclide). The alpha particle removes two protons (green) and two neutrons (gray) from the uranium-238 nucleus.
3.1 Major Forms of Radioactivity
Alpha Particle (α)
Rutherford’s experiments demonstrated that there are three main forms of radioactive emissions. The first is called an alpha particle, which is symbolized by the Greek letter α. An alpha particle is composed of two protons and two neutrons and is the same as a helium nucleus. (We often use 24He to represent an alpha particle.) It has a 2+ charge. When a radioactive atom emits an alpha particle, the original atom’s atomic number decreases by two (because of the loss of two protons), and its mass number decreases by four (because of the loss of four nuclear particles). We can represent the emission of an alpha particle with a chemical equation—for example, the alpha-particle emission of uranium-235 is as follows:
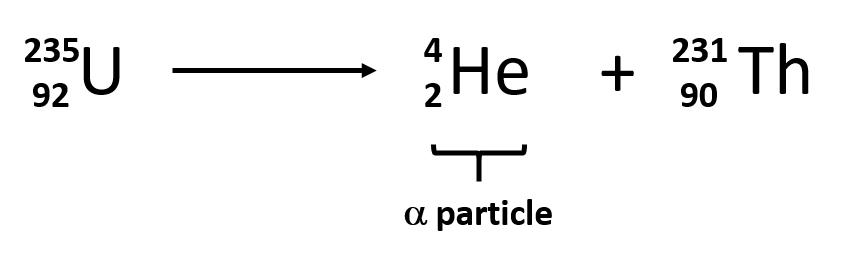
Rather than calling this equation a chemical equation, we call it a nuclear equation to emphasize that the change occurs in an atomic nucleus. How do we know that a product of this reaction is 90231Th? We use the law of conservation of matter, which says that matter cannot be created or destroyed. This means we must have the same number of protons and neutrons on both sides of the nuclear equation. If our uranium nucleus loses 2 protons, there are 90 protons remaining, identifying the element as thorium. Moreover, if we lose four nuclear particles of the original 235, there are 231 remaining. Thus we use subtraction to identify the isotope of the Th atom—in this case, 90231Th.
Beta Particle (β)
The second type of radioactive emission is called a beta particle, which is symbolized by the Greek letter β. A beta particle is an electron ejected from the nucleus (not from the shells of electrons about the nucleus) and has a -1 charge. We can also represent a beta particle as -10e. The net effect of beta particle emission on a nucleus is that a neutron is converted to a proton. The overall mass number stays the same, but because the number of protons increases by one, the atomic number goes up by one. Carbon-14 decays by emitting a beta particle:
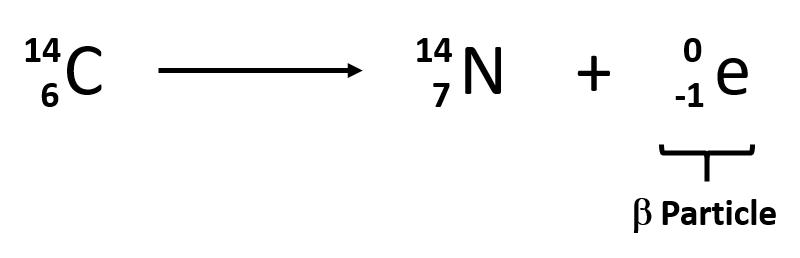
Again, the sum of the atomic numbers is the same on both sides of the equation, as is the sum of the mass numbers. (Note that the electron is assigned an “atomic number” of -1, equal to its charge.)
Gamma Radiation (γ)
The third major type of radioactive emission is not a particle but rather a very energetic form of electromagnetic radiation called gamma rays, symbolized by the Greek letter γ. Electromagnetic radiation can be characterized into different categories based on the wavelength and photon energies. The electromagnetic spectrum shown in figure 3.2 shows the major categories of electromagnetic radiation. Note that the human sensory adaptations of sight and hearing have evolved to detect electromagnetic radiation, with radio waves having wavelengths between 1 mm and 100 km and visible light having wavelengths between 380 – 700 nm. Technological advances have helped humankind utilize other forms of electromagnetic radiation including X-rays and microwaves.
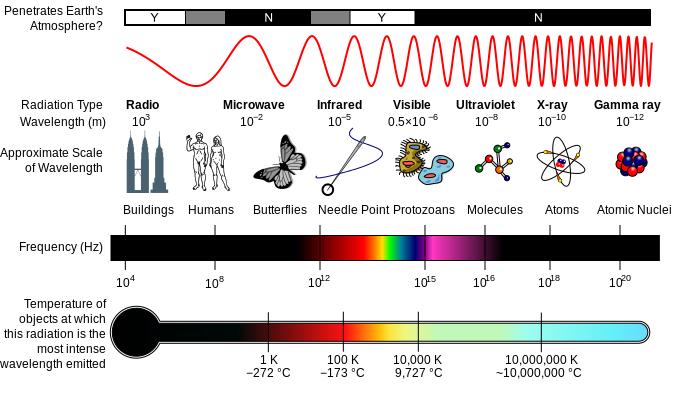
Figure 3.2 The Electromagnetic Spectrum. A diagram of the electromagnetic spectrum, showing various properties across the range of frequencies and wavelengths. Image Available from Wikipedia
Some electromagnetic radiation with very short wavelengths are active enough that they may knock out electrons out of atoms in a sample of matter and make it electrically charged. The types of radiation that can do this are termed ionizing radiation. X-rays and Gamma rays are examples of ionizing radiation. Some radioactive materials, emit gamma radiation during their decay. For example, in the decay of radioactive technetium-99, a gamma ray is emitted. Note that in radioactive decay where the emission of gamma radiation occurs, that the identity of the parent material does not change, as no particles are physically emitted.
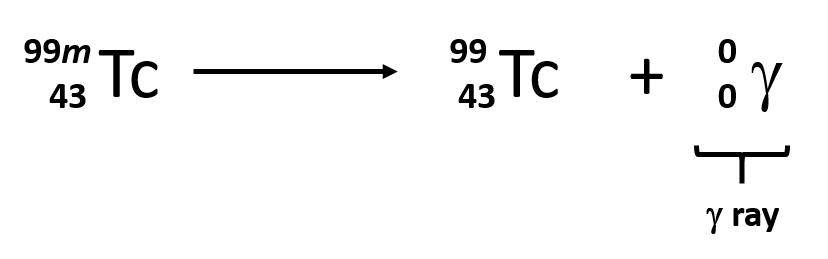
Sometimes the radioactive decay of a sample can result in the release of multiple forms of radioactivity. For example, in the radioactive decay of radon-222, both alpha and gamma radiation are emitted, with the latter having an energy of 8.2 × 10−14 J per nucleus decayed:
Read more : Which Of The Following Turfgrasses Has The Least Shade Tolerance
This may not seem like much energy, but if 1 mol of Rn atoms were to decay, the gamma ray energy would be 4.9 × 107 kJ!
Alpha, beta, and gamma emissions have different abilities to penetrate matter. The relatively large alpha particle is easily stopped by matter (although it may impart a significant amount of energy to the matter it contacts). Beta particles penetrate slightly into matter, perhaps a few centimeters at most. Gamma rays can penetrate deeply into matter and can impart a large amount of energy into the surrounding matter. Table 3.1 summarizes the properties of the three main types of radioactive emissions and Figure 3.3 summarizes the ability of each radioactive type to penetrate matter.
where 01n is a neutron. As with any nuclear process, the sums of the atomic numbers and mass numbers must be the same on both sides of the equation. Spontaneous fission is found only in large nuclei. The smallest nucleus that exhibits spontaneous fission is lead-208. (Fission is the radioactive process used in nuclear power plants and one type of nuclear bomb.)
(Back to the Top)
3.2 Radioactive Half Lives
Each radioactive nuclide has a characteristic, constant half-life (t1/2), the time required for half of the atoms in a sample to decay. An isotope’s half-life allows us to determine how long a sample of a useful isotope will be available, and how long a sample of an undesirable or dangerous isotope must be stored before it decays to a low-enough radiation level that is no longer a problem.
For example, cobalt-60, an isotope that emits gamma rays used to treat cancer, has a half-life of 5.27 years (Figure 3.5). In a given cobalt-60 source, since half of the nuclei decay every 5.27 years, both the amount of material and the intensity of the radiation emitted is cut in half every 5.27 years. Note that for a given substance, the intensity of radiation that it produces is directly proportional to the rate of decay of the substance and the amount of the substance. Thus, a cobalt-60 source that is used for cancer treatment must be replaced regularly to continue to be effective.
Since every half-life for a radionuclide is the same length of time, we can use the following equation to calculate how much radioactive nuclide is remaining after the passage of any number (n) of half-lives:
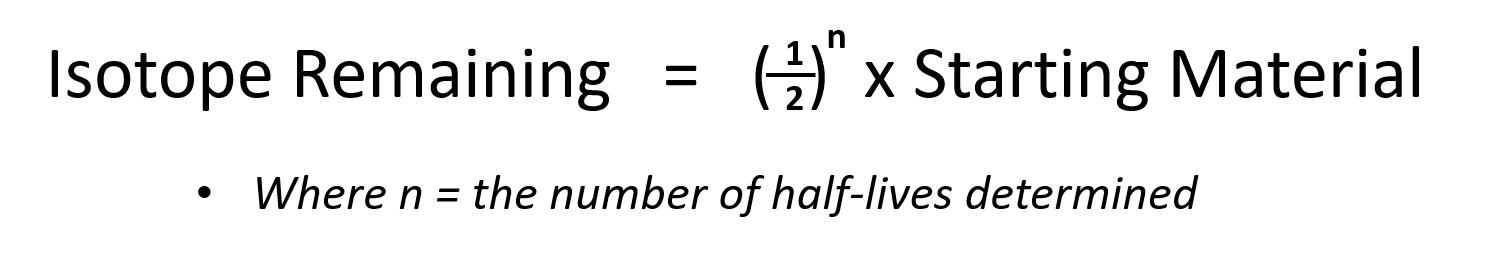
There is a large difference in the magnitude of the biological effects of nonionizing radiation (for example, light and microwaves) and ionizing radiation, emissions energetic enough to knock electrons out of molecules (for example, α and β particles, γ rays, X-rays, and high-energy ultraviolet radiation) (Figure 3.6).
Energy absorbed from nonionizing radiation speeds up the movement of atoms and molecules, which is equivalent to heating the sample. Although biological systems are sensitive to heat (as we might know from touching a hot stove or spending a day at the beach in the sun), a large amount of nonionizing radiation is necessary before dangerous levels are reached. Ionizing radiation, however, may cause much more severe damage by breaking bonds or removing electrons in biological molecules, disrupting their structure and function (Figure 3.7).
Source: https://t-tees.com
Category: WHICH