Patients with cancer at the end of life can have altruistic goals, such as having a life that mattered, being part of a larger existential self, feeling free to express themselves, using unrestrained and honest talk, giving back to society, and reconciling and integrating themselves into complete human beings who are engaged in the world (Bloomer et al., 2018). A systematic review by Alexander et al. (2018) reported that patients at the end of life viewed participating in research studies as worthwhile, with the positive benefits outweighing any negative risks, such as an increase in symptoms. According to Wohleber et al. (2012), denying this vulnerable population the right to participate in intervention research studies would also deny their autonomy and show a lack of respect for their humanity.
Patient-Related Barriers and Comorbidities
Cancer progression, preexisting conditions, and end-of-life symptoms are some factors that can prevent seriously ill patients from enrolling in intervention studies. Depending on the patient’s cancer stage and comorbidities, common physical symptoms at the end of life include pain, dyspnea, congestion, edema, ascites, and nausea and vomiting (Stark et al., 2012). Possible psychological symptoms at the end of life can include anxiety, depression, confusion, diminished responsiveness, hallucinations, and delirium (Stark et al., 2012). On average, older adult patients with cancer have at least five comorbid conditions (Hamaker et al., 2018). Comorbid conditions can negatively influence patients’ physical and psychological well-being at the end of life. Although the deterioration of a patient’s physical and mental faculties is inevitable, the timing of this decline is unpredictable. The intensity of the patient’s deteriorating condition and the level of caregiver strain and burden on family members have been cited as primary reasons for patients deciding not to participate in research studies (Kaasa et al., 2018).
You are viewing: Which Situation Is A Common Barrier To Hospice Care
Gatekeeper Perspective
Access to patients may be limited by family members or caregivers, who can also be known as gatekeepers (Walter & Read, 2011). The patient’s gatekeeper may be his or her family member or a member of the palliative care team, hospice ethics committee, or the interprofessional healthcare team. Research involving patients at the end of life was thought to be damaging, insensitive, and intrusive, and these ethical concerns have been evaluated to determine whether such clinical research exploits an already vulnerable patient population (Walter & Read, 2011). In a systematic review of 30 studies on gatekeeping in palliative care research, Kars et al. (2016) found that the fear of burdening patients is the most commonly reported reason for gatekeeping. Gatekeepers at the bedside have also expressed concerns about their involvement in clinical research when determining prognosis to meet eligibility criteria, approaching patients as the recruiter for the study, and scheduling a time for patients and the researcher to meet (Melnyk et al., 2018; Whitehead & Clark, 2016).
Researcher Perspective
Conducting research that involves patients at the end of life requires overcoming the challenges and barriers associated with hospice and palliative care, which is often described as a herculean feat (Hanson et al., 2014). Multiple factors may contribute to the complexity of performing research with patients and family members in this population, including recruitment, attrition, prognostication, physical and psychological symptoms, and study logistics.
Read more : Which Graph Represents The Compound Inequality
Goldberg (2004, 2005) cited recruitment and attrition as the primary barriers to research on hospice and palliative care. Patients may be deterred from participating in an intervention research study if they are in denial of their advancing disease or if they do not acknowledge their mortality. Patients or their family members may be overwhelmed by the terminal prognosis. About 1.2 million patients have participated in hospice and end-of-life care-funded initiatives, but intervention studies are limited because it is difficult to find methodologic approaches that have been proven to be successful in completing research studies involving these patients (Hanson et al., 2014).
Overestimation of prognosis (White et al., 2016), poor prognostic tools (Kaasa et al., 2018), and difficulty having end-of-life conversations (Waldrop & Meeker, 2012) may affect the volume of hospice referrals and the length of patients’ stays in hospice care. White et al. (2016) identified a disparity between patients’ life expectancy and physicians’ estimates for prognosis, which can make it difficult to identify patients with cancer who may meet study inclusion criteria. In a 2012 study, Wohleber et al. found that 51% of patients died during the first month following a referral to hospice. According to the National Hospice and Palliative Care Organization (2019), it is common to have as many as 28% of patients die within a week of their admission to hospice.
Methodologic Issues
Previous studies have suggested that study criteria are too stringent or restrictive, preventing the enrollment of possible participants (Goldberg, 2005; Kutner et al., 2005). Maximizing the volume of eligible patients through less restrictive eligibility criteria can provide a larger pool of potential participants for enrollment. Securing research funding has also been identified as a challenge. Using a study design that may not be appropriate or acceptable for dying patients (e.g., a randomized controlled trial), having multiple data collection points, and requiring patients to complete frequent paperwork or surveys or to participate in lengthy interviews can be burdensome as well (Kutner et al., 2005). Hagen et al. (2011) advocated for reducing patient burden by simplifying the wording of informed consent documents, eliminating extraneous information, and altering the delivery of the informed consent requests (e.g., verbal instead of written). Limiting the number of data collection points and streamlining data collection procedures can also help to minimize patient burden. In addition, study procedures can be simplified with an energy-conserving consent process that reduces the amount of time spent to acquire patient consent or gives patients the option to provide consent when their symptom burden and fatigue levels are likely to be lower and their energy levels are higher (e.g., on a weekend).
Implications for Nursing
Hospitals that have earned Magnet designation have been a catalyst for engaging frontline nurses with intervention research. The role of bedside oncology nurses starts well before the first patient is enrolled in the intervention study, with collaboration during the creation of the study. With their knowledge of the research process, their use of evidence-based research findings in practice, their skills in communication and ability to evaluate eligibility, and an attitude of appreciation and value toward research findings, bedside clinicians can become valuable components of the research process. As patient advocates, nurses can adopt an urgent response reaction when patients decide to participate in intervention studies and can coordinate with the research and discharge teams to optimize patient outcomes.
Read more : Which Welding Rod To Use
It is important to the success of intervention studies that researchers understand organizational structure and clinical operations at the study setting to use the best strategies for patient accrual. Building a relationship with organizational gatekeepers, institutional review board members, bedside clinicians, and any other individuals essential to the success of the study is also important. To minimize time constraints and burdens for bedside nurses, researchers can collaborate with nurse informatic specialists to create an eligibility report from data in the electronic health record (EHR). Checks and balances that are built into the study intervention provide a means to measure the consistency of the researchers and the proposed processes. Researchers can involve patients and gatekeepers in the process of developing an operational implementation plan for the study that considers the flow of the bedside process.
Access to accurate and timely information from the EHR regarding patients’ conditions is instrumental in conducting a successful research study, and nurse gatekeepers can inform researchers of any psychological symptoms or impaired cognitive function being experienced by patients that would prohibit enrollment (Brohard, 2017). Involving nurse gatekeepers in recruitment, data collection, and implementation may require training with frequent, regular reliability checks to identify and resolve potential issues. Written procedures and checklists associated with screening, enrollment, consent, intervention, and evaluation can ensure that all study aims are accomplished. In a previous study, complete participation was achieved (zero attrition) by reducing the chance of dropout from patient-related barriers (e.g., physical or psychological symptoms) with ongoing monitoring via the EHR, minimizing study-specific barriers with efficiency built into the study design, and incorporating procedures, such as a five-day waiting period, electing an urgent response system, and considering an one-encounter intervention (Brohard, 2017).
Successful completion of hospice intervention research can be achieved with the resolution of barriers and challenges specific to this specialty research area. Figure 1 summarizes barriers to intervention studies conducted in a palliative care setting, with recommendations for resolution. 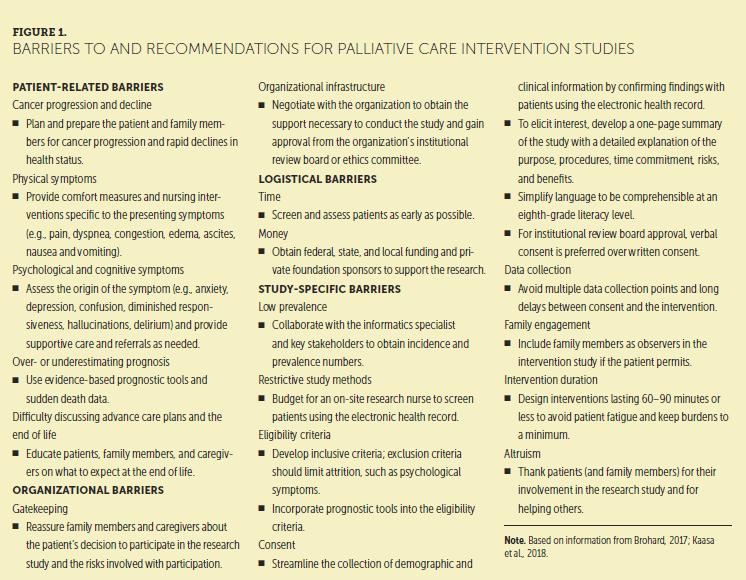
Conclusion
The complexity of planning, funding, and completing an intervention study cannot overshadow the importance of conducting these studies to support the palliative care needs of patients. The findings of hospice and palliative care intervention research studies are needed to provide evidence-based end-of-life care, improve death and dying policies, and support caregivers coping with the burdens of caring for a dying patient. To successfully complete end-of-life nursing intervention research studies, strategic planning is needed to address the common barriers that might be encountered. Nurse researchers can change the architecture of hospice research, accept the inherent limitations of the hospice and palliative care population, embrace the richness of the research and its findings, and alter approaches for patient-centered models. Conducting nursing intervention research can improve the care of patients at the end of life and provide important data for developing evidence-based quality hospice care.
About the Author(s)
Cheryl Brohard, PhD, RN, CNS-ONC, AOCN®, CHPCA®, is an assistant professor in the College of Nursing at the University of Houston in Texas. The author takes full responsibility for this content and did not receive honoraria or disclose any relevant financial relationships. Brohard can be reached at [email protected], with copy to [email protected].
Source: https://t-tees.com
Category: WHICH
