Click here to view as a pdf: Understanding Biofilms In Agriculture Customer Favorite1
Customer Favorite This article was originally published in the April 2017 Issue of the Crystal Creek® Newsletter
You are viewing: Why Are Empty Bleach Buckets Unacceptable
By: Jessica Getschel, B.S.
In agriculture today, sanitation technique and protocol implementation have become more important than ever before. An increased awareness of health benefits gained from a clean environment has stimulated a higher standard of cleaning expectations. Many producers not only strive to remove organic matter from surfaces, but also microbial buildup; more accurately, biofilms.
What Are Biofilms?
Biofilms are simply defined as thin, slimy films of bacteria, protozoa and viruses adhered to a surface in a resistant matrix of cellular materials. Biofilm layers are found on many farm surfaces such as feeding equipment, animal housing and milking equipment. Roughly 90% of all bacteria on a farm are found in a biofilm layer. These biofilm layers are important because they are resistant to common cleaning and disinfection agents. To truly clean a surface, one must break down the biofilm layer to achieve not only a visually clean surface, but a surface that is also clean on a biological level.
How Do Biofilms Form?
In the past decade many researchers have investigated the process of biofilm formation. It has been well established that there are five major steps comprising the entire process: attachment, growth, maturation, detachment, and re-development. Figure 1 illustrates the cycle of biofilm formation.
Why Are Biofilms Important?
Read more : Why Is My Vizio Tv Not Connecting To Wifi
Biofilms have potential to be detrimental in the agriculture industry because of the opportunity for cross-contamination. Equipment and pens that are visually clean may not be biologically clean. Biofilms limit the rate of cleaning and disinfecting agents to the interior cells while providing conditions for those same cells to thrive1. These cells can be disease-causing bacteria that can spontaneously break free from the biofilm and spread sickness to an animal. One example would be placing a newborn calf in a hutch that previously housed a weaned calf. Any bacteria harbored by the older calf could be contained in a biofilm and may not have been removed during the hutch cleaning process. In this scenario, the bacteria could break free from the biofilm and pose a serious health challenge to the newborn calf.
How Are Biofilms Broken Down?
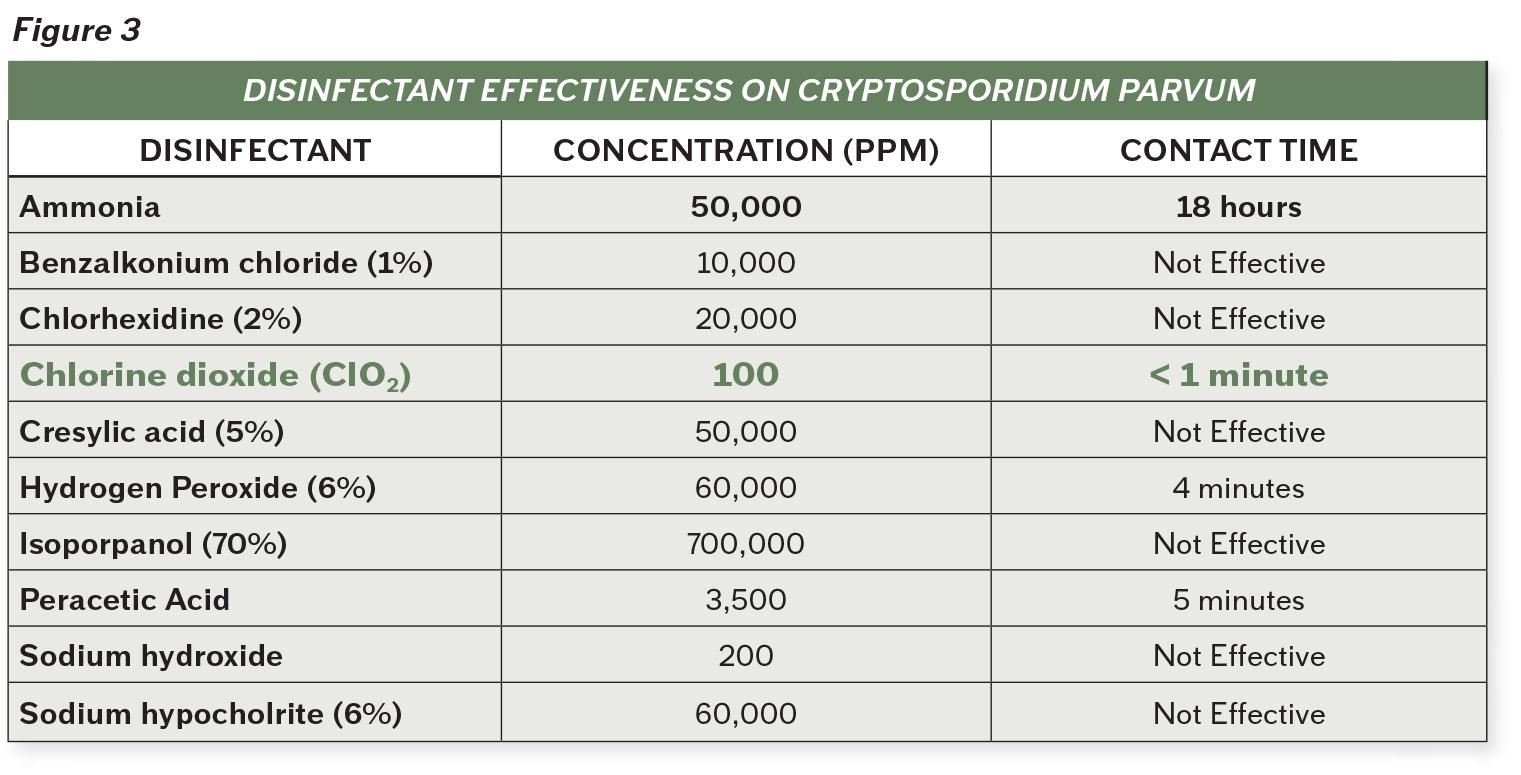
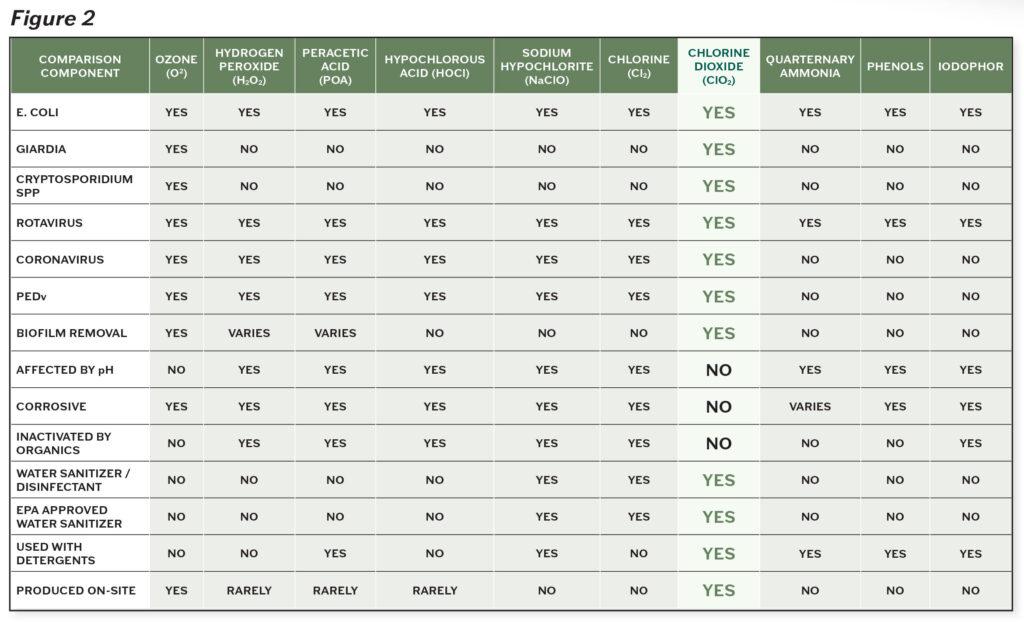
There are very few products proven to be effective against the tough buildup of biofilms. Figure 2 demonstrates the efficacy of various products on biofilms, while comparing microbial diversity and environmental considerations.
The clear standout agent is chlorine dioxide. Chlorine dioxide has superior ability to break down the toughest microorganisms and biofilms, without corrosive action or negative impacts on the environment. Its efficacy is not impacted by the condition of the environment, most notably in regards to pH levels and presence of organic matter. Chlorine dioxide is effective against bacteria, protozoa, viruses and fungi on inanimate objects and is considered more effective against microbes than other chlorine solutions2. Unlike other cleaning products, chlorine dioxide starves and kills microorganisms by disrupting the transport of nutrients across their cell walls2.
Chlorine dioxide is even effective against Cryptosporidium, a tough protozoan responsible for causing diarrhea in many different livestock, most notably calves. According to the CDC, this organism has an outer shell that allows it to survive without a host for long periods of time and makes it very tolerant to bleach disinfection.
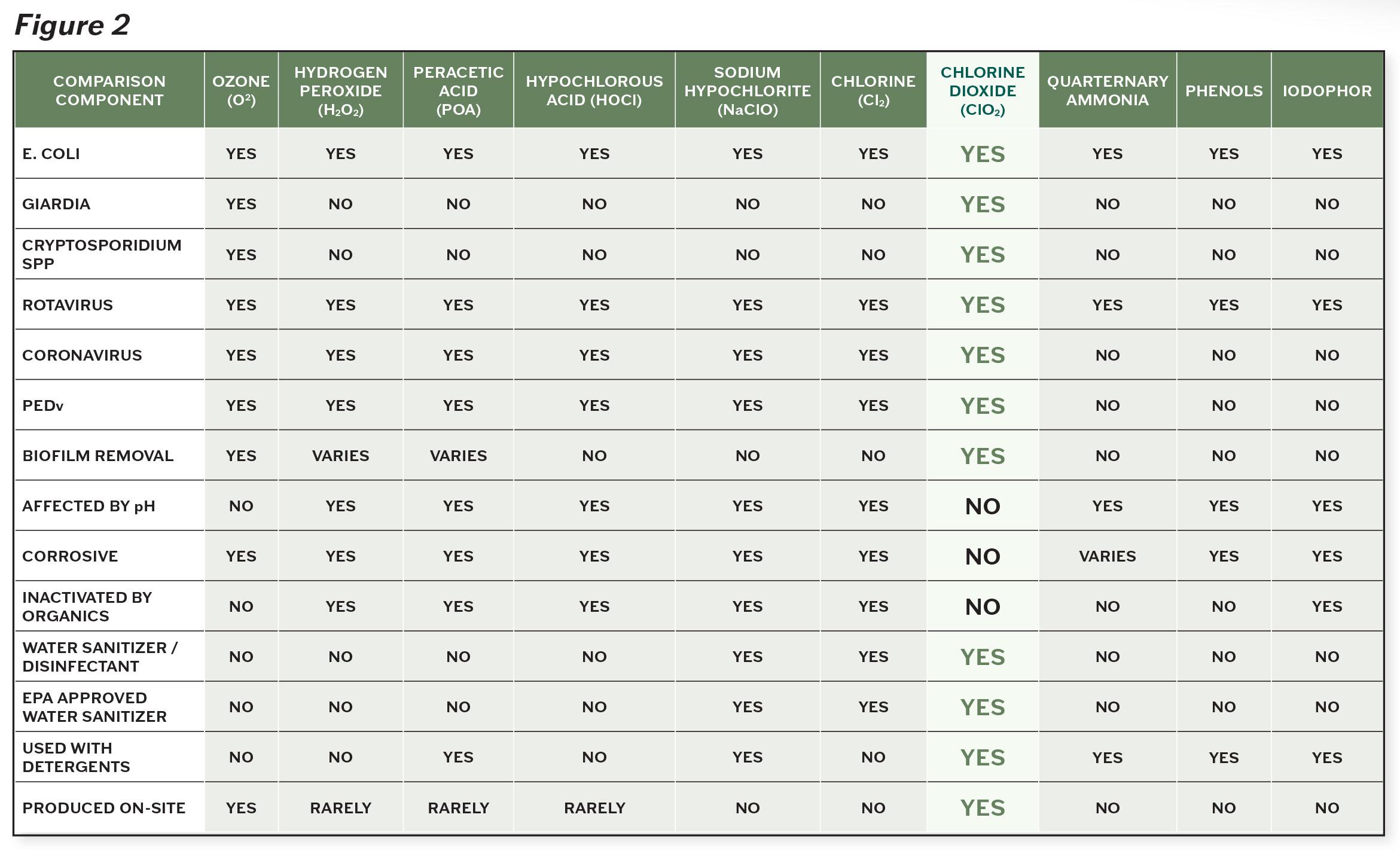
Read more : How to Fix the Issue of Words Going Off the Page in Google Docs
As demonstrated by the chart above, chlorine dioxide is clearly the product of choice when dealing with cryptosporidium. With less than a minute of contact time, chlorine dioxide can incapacitate the microorganism at a much lower concentration compared to other products.
Why Not Use Household Bleach?
The efficacy of bleach is determined by the pH of the mixed solution. When mixed with water, bleach (sodium hypochlorite) breaks into two compounds: hypochlorous acid and hypochlorite ion. Hypochlorous acid has about 80 times the killing power of hypochlorite ion and is minimally present in solutions with a pH of 10 or greater. A 10% bleach solution has a pH of 10-11 and therefore has a greatly reduced ability to effectively perform as a sanitizing agent.3
How Can Biofilm Awareness Be Raised?
One way to locate the unseen biofilms is to test for them. An ATP meter can be used to identify areas of high microbial activity and can also be used to monitor and evaluate the effectiveness of a cleaning protocol. Swabs are taken from materials that are cleaned, such as nipples, buckets, panels or equipment. The ATP meter then provides a numerical readout that will reveal the efficacy of the cleaning protocol in place. If the meter readings indicate unacceptable levels of microbial activity, it is advised that the cleaning protocol be reevaluated.
Regardless of the operation (e.g., dairy, swine, poultry) all livestock producers can benefit from biofilm reduction. Reducing livestock exposure to pathogens will decrease mortality and sickness rates, thereby decreasing treatment costs and increasing profitability. Biofilm buildup is a serious issue that should be heavily considered when selecting a sanitizing agent. For ways to prevent biofilm buildup and to improve your cleaning protocol, see Erik Brettingen’s article in the April 2017 Crystal Creek® Newsletter issue titled “Hygiene Protocols For Succcessful Calf Raising”. 
Sources:
- Donlan, Rodney M. “Biofilm Formation: A Clinically Relevant Microbiological Process.” Clinical Infectious Diseases 33.8 (2001): 1387-392. Web.
- Valderrama, W. B., E. W. Mills, and C. N. Cutter. “Efficacy of Chlorine Dioxide against Listeria monocytogenes in Brine Chilling Solutions.” Journal of Food Protection 72.11 (2009): 2272-277. Web.
- Socket, Donald C. “6 Easy Steps to Properly Clean and Sanitize Calf Feeding Equipment” (2012). Web.
Source: https://t-tees.com
Category: WHY
