Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterised by multiorgan involvement and autoantibody production.1 As a chronic disease, lupus has a phase of remission and relapse.2 Lupus has a broad spectrum of clinical manifestations.3 The presence of immune complex deposition and the binding of autoantibodies to various tissues cause damage to these tissues. This damage can occur in almost all organs of the body, with varying clinical manifestations. The most prominent damage occurs in the skin, joints, kidneys and serosal membranes (such as the peritoneum, pericardium and pleura).2
Patients with SLE have higher mortality and morbidity rates than the general population. The leading cause of death in lupus patients is cardiovascular disorders.4,5 Dyslipidaemia is known to be one of the main risk factors for cardiovascular disorders and the main cause of death in patients suffering from SLE.6,7 Dyslipidaemia is a condition in which the concentration of lipids in the blood is abnormal and is common in SLE patients. This usually involves increased levels of total cholesterol, triglycerides and LDL and a decreased level of HDL.4,5
You are viewing: Why Do Steroids Increase Cholesterol
A cohort study of 918 SLE patients from Systemic Lupus International Collaborating Clinics found that 33% of newly diagnosed patients had hypertension and 36% of them had hypercholesterolaemia.4 In Asia, the prevalence of dyslipidaemia in SLE patients was found to be very high, reaching up to 65.3-84.6% of total patients, with an increase of total cholesterol by 43%, increase of LDL level by 26.4%, increase of triglycerides by 44.2% and decrease of HDL by 26%.8
Previous studies showed that many factors play a role in the development of dyslipidaemia in SLE patients, such as autoantibodies, cytokines and the treatment of lupus itself (steroids and cyclosporine A). Those studies also showed that corticosteroids induce dyslipidaemia in SLE patients in a dose-dependent manner.4,8 Steroids are known to have side effects on metabolism and endocrine function that can cause metabolic changes of carbohydrates, proteins and lipids.9-11 Steroids stimulate the conversion of proteins into carbohydrates through gluconeogenesis and stimulate the storage of carbohydrates as glycogen. Large amounts of steroids can cause the redistribution of fat to the upper body and face.9,10
Several studies have also shown that steroid therapy could affect the lipid profile.8-11 However, there is still little information about its effects in SLE patients. We thus conducted this study to determine the correlation between steroid therapy and lipid profiles in SLE patients.
Methods
Study Design and Patients
We performed a correlative analytic study with a cross-sectional design by collecting retrospective data from medical records. The minimum sample required 38 subjects with the significance levels of alpha (α) = 5%, beta (β) = 10% and correlation coefficient (r) of 0.5. The patients’ data were selected by simple random sampling from Hasan Sadikin Lupus Registry (HSLR) from 2008 to 2019.12 The population of this study was all SLE patients at the rheumatology outpatients clinic of the Internal Medicine Department at Dr. Hasan Sadikin Hospital, Bandung. This research was conducted by collecting secondary data from HSLR and patients’ medical records.12 The inclusion criteria of this study were patients who had undergone a lipid profile examination, been treated with steroids and registered in the Hasan Sadikin Lupus Registry. The exclusion criteria for this study were SLE patients taking cyclosporine A, statins, patients receiving steroids for less than one year and those with other diagnoses or habits that affect the lipid profile, such as diabetes mellitus, smoking and hypothyroidism.
The variables assessed in this study were steroid dose as an independent variable and parts of the lipid profile (total cholesterol, LDL, HDL and triglycerides) as dependent variables. The average daily steroid dose is defined as the steroid dose in the past year since the patient’s lipid profile was examined. This study also assessed characteristics of the subjects such as gender, age, length of time since diagnosis, and the presence or absence of kidney involvement (kidney disorders).
This study was reviewed and approved by the Research Ethics Committee of Padjadjaran University (ethical clearance number 733/UN6.KEP/EC/2019) and was conducted in compliance with the Declaration of Helsinki. All patients provided written informed consent for the data transfer of their medical records to the HSLR.
Statistical Analysis
The data that had been collected were then processed and analysed using IBM SPSS Statistics version 20.0. The descriptive analysis was performed first to show the characteristics of patients. Normality of the data was then analysed using the Shapiro-Wilk test to determine the distribution of various variables. When appropriate, data were transformed using logarithmic transformation. The correlations between steroid dose and lipid profiles were analysed with Pearson’s correlation test.
Results
From the 82 patients who met the inclusion and exclusion criteria, 41 patients were then randomised as subjects. The patients were all female, with an average age at diagnosis of 30 ± 9.29 years. Seven patients had suffered from SLE for less than three years and 34 patients had done so for more than three years. There were 21 (51.2%) patients with kidney disorders in this study (Table 1).
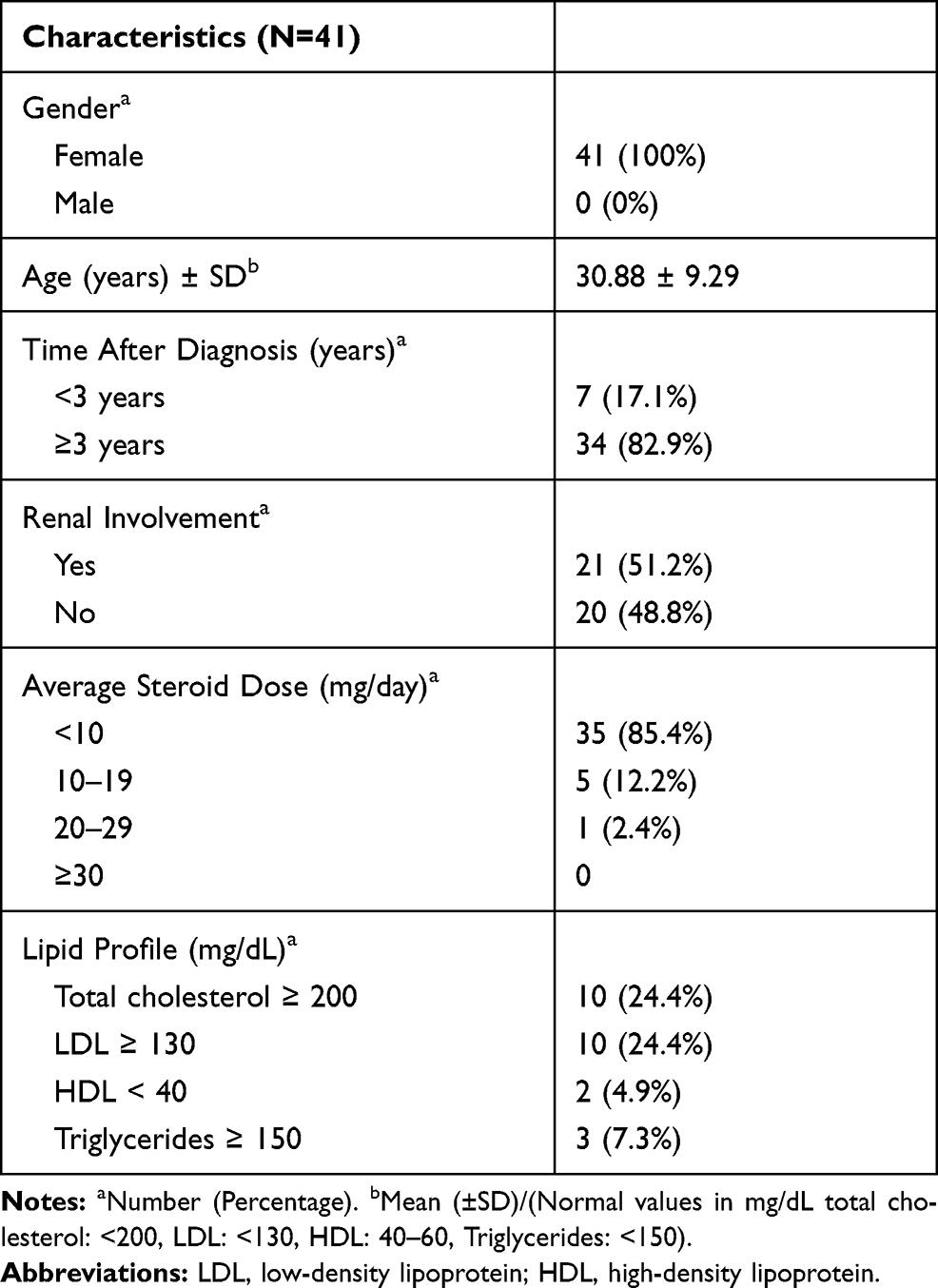
Table 1 Demographics, Disease Characteristics and Lipid Profile in SLE Patients
There were 35 patients (85.4%) who had an average steroid dose below 10 mg/day, classified as a low steroid dose, five patients (12.2%) with a moderate steroid dose of 10-19 mg/day, and only 1 (2.4%) patient with a high steroid dose of 20-29 mg/day, as shown in Table 1. We then categorised the steroid dose data according to a previous paper. The dose was categorised as low if it was less than 2.5 mg/dL, moderate or medium if it was 2.5-7.5 mg/dL and high if it exceeded 7.5 mg/dL.13 According to this categorisation, we showed that the highest dose of steroid therapy was 21.37 mg/day and the lowest steroid dose was 0.65 mg/day (Table 2). The average steroid dose in this study was 5.64 mg/day. In terms of the lipid profile, the average levels were 177.51 mg/dL for total cholesterol, 105.22 mg/dL for LDL, 61.00 mg/dL for HDL and 92.98 mg/dL for triglycerides (Table 3). Some patients had lipid levels above the normal limit, namely, ten patients for total cholesterol levels, ten patients for LDL levels, two patients for HDL levels and three patients for triglyceride levels.
Table 2 Steroid Therapy Classification in SLE Patients
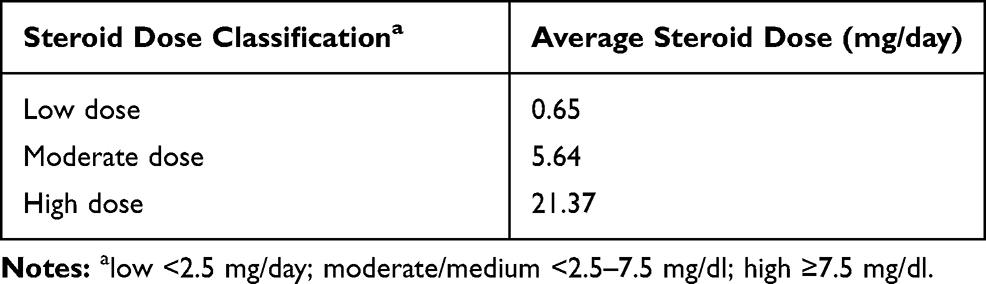
Table 3 Lipid Profile in SLE Patients
The test of the correlation between steroid dose and total cholesterol showed an r-value of 0.375 with a p-value of <0.05, which means that there was a significant but weak correlation. The test between steroid dose and LDL showed an r-value of 0.308 with a p-value of >0.05, while for HDL there was an r-value of 0.206 with a p-value of >0.05, indicating the lack of a significant correlation between those variables. The test of the correlation between steroid dose and triglycerides showed an r-value of 0.416 with a p-value of <0.05, indicating a significant correlation of moderate strength (Table 4).
Table 4 Correlation Coefficients Between Steroid Therapy and Lipid Profile
Additionally, we analysed the correlation between ages of the subjects and the lipid profile. A previous study showed that different lipid levels were observed in patients of various age groups.14 Further, the nonelderly patients tended to have higher lipid levels than elderly patients. However, within this study, we did not find any correlation between age and lipid profile (data not shown).
Discussion
The incidence of SLE varies greatly from country to country, ranging from 0.3 to 23.7 per 100,000 people/year, while the prevalence ranges from 6.5 to 178 per 100,000 people. Systematic studies in the Asia-Pacific region also showed an incidence of 0.9 to 3.1 per 100,000 people/year, with a prevalence of around 4.3 to 45.3 per 100,000 people.15
Read more : Why Is Crime So High In Billings Montana
The results of this study showed that all of the patients were female (100%) with an average age at diagnosis of 30.88 ± 9.29 years. The results were similar to those in previous studies that stated that SLE mostly affected women of reproductive age rather than men, at a ratio of 22:1.2,16 Studies in Indonesia showed that the age of SLE patients ranged between 31 and 40 years.6 Another study also showed an average age at SLE diagnosis of 27.7 ± 9.4 years.12 The incidence of SLE is associated with changes in hormones that occur during the growth period, such as puberty.6 Most patients had suffered for SLE for more than three years. This result is similar to that in a previous study showing that 82.9% of SLE patients had had the disease for more than three years. In addition, studies have shown that the prevalence of dyslipidaemia in lupus patients ranges from 36% to 60% at the time of diagnosis, and even higher after three years.8,17
The present study showed that a total of 21 patients (51.2%) had renal disorders. SLE patients can experience skin, kidney, haematological and musculoskeletal involvement.18 The patients with kidney disorders are known to have signs of nephrotic syndrome, such as proteinuria. It has also been found that dyslipidaemia is common in lupus nephritis patients and it is strongly associated with proteinuria due to the increase in lipid synthesis.6,17
Our recent study found that the average steroid dose of subjects did not exceed 30 mg/day. However, another study found that 74% of patients had a steroid dose of less than 30 mg/day.6 It showed that patients who took corticosteroid with a dose of more than 30 mg/day experienced significant increases in total cholesterol and triglycerides.6,8
The average lipid profile here showed no significant increase, except for HDL with an increase of 61.00 mg/dL (SD ± 14.99). There were abnormalities of lipid profile in terms of total cholesterol (≥ 200 mg/dL) by 24.4%, LDL (≥ 130 mg/dL) by 24.4%, HDL (<40 mg/dL) by 4.9% and triglycerides (≥ 150 mg/dL) by 7.3%. A cohort study in Brazil showed that, among 185 SLE patients, 48% had hypercholesterolaemia, 30% had hypertriglyceridaemia and 60% experienced both. In Asia, dyslipidaemia in SLE patients was found at rates of 65.3% to 84.6%, with an increase in total cholesterol by 43%, LDL levels by 26.4% and triglycerides by 44.2% and a decrease in HDL by 26%.8 The difference in these results could be caused by the different sample populations, characteristics and methods involved.
As a comparison with healthy Indonesian subjects, a previous paper showed the level of total cholesterol of 193.17 mg/dL, with 130.22 mg/dL for LDL and 43.20 mg/dL for HDL. However, in this study, subjects aged 18 or older were recruited, with a greater variety of subjects. Finally, it was concluded that age and ethnicity affect the risk of dyslipidaemia in Indonesia. In this previous study, the dietary pattern of each ethnic group in Indonesia was also discussed. However, there were differences in the inclusion criteria related to LDL and HDL levels of the subjects compared with the current study.19
Steroid, which is used in SLE therapy, is known to cause lipid profile abnormalities, such as increases in total cholesterol, triglyceride and LDL and a decrease in HDL levels.4-6,20 Similar results were seen in a previous study. It was found that total cholesterol and triglyceride levels were increased in SLE patients who had steroid therapy.21 Although the exact mechanism by which steroids affect the lipid profile is not yet known, steroids are known to influence fat metabolism, such as increased lipolysis, increased lipoprotein lipase (LPL), increased adipokine activity, increased insulin resistance and free fatty acid β-oxidation inhibition.8 Besides steroid, some drugs such as anti-hypertension drugs (diuretics, β-blocking agents), oral contraceptives (progestogens), immunosuppressive agents (cyclosporine, azathioprine) and hydroxychloroquine affect the lipid profile.22,23 Although we did not screen all therapies that could affect the lipid profile in the subjects, we did exclude the subjects who had been treated with cyclosporine and statin.
We found that there was a weak positive correlation between steroid dose and total cholesterol. However, there was a moderately strong positive correlation between steroid dose and triglycerides. Steroids play a role in the regulation of triglyceride-dependent homeostasis that relies on physiological conditions, to modulate the synthesis and hydrolysis of triglycerides. It can affect both processes in adipocytes. A study by Wang et al showed that glucocorticoid-regulated genes, especially primary potential target glucocorticoid receptor genes, are involved in triglyceride metabolism.24
Steroids cause changes in lipoprotein metabolism through hypercortisolism, which causes insulin resistance and hyperinsulinemia, and subsequently stimulates the production of very low-density lipoprotein (VLDL) and HDL. In vitro studies have also shown that steroids inhibit LDL uptake and internalisation, but they can also interfere with VLDL catabolism through decreased LPL activity.6 A previous study assumed that an increase of VLDL hydrolysis after steroid treatment could increase the HDL levels. The short-term administration of steroids will increase the activity of lecithin-cholesterol acyltransferase (LCAT), which will eventually increase HDL2. Conversely, long-term corticosteroid administration will suppress HDL2 levels.6 However, our study showed that steroid has a weak influence on LDL and HDL, as indicated by the low r-values. These results indicate that no significant correlation was found between the factors. This could be due to wide variations in individual responses to steroids or variations in lipoprotein metabolism.
A limitation of this study was the lack of data on baseline lipid levels before the administration of steroid therapy. This study also did not consider the possible confounding factors such as a history of kidney disorders and SLE disease activity index (SLEDAI) scores.
Further studies should be conducted on a larger population and consider the confounding factors that might influence the results, including the disease activity, to ensure that the findings represent the actual conditions.
Conclusion
We reveal a statistically significant positive but weak correlation between steroid dose and total cholesterol, and a moderate correlation between steroid dose and triglycerides, but no significant correlation between steroid dose and either HDL or LDL.
Source: https://t-tees.com
Category: WHY
